The Cheesy Energy of Lithium
An Exploration of Lithium's Power in Batteries, Medicine, and Nuclear Physics
Table of Contents
- Curiosity = Love
- How to Read This Book
- Lithium: An Atom's Perspective
- "I'm radioactive, radioactive": The Magic of Radioactive Decay and the Charms of Radioactivity
- A Journey to the Subatomic World: Theoretical Physics, Particles, and Matryoshkas
- Properties of Lithium
- History of Lithium
- Lithium's Applications: Batteries, Drugs, and Atomic Bombs
- Nuclear Physics: Lithium-6, Fusion, Reactors, and The Future
- Lithium-Ion Batteries
- Batteries Explained
- The History of the Battery
- History of the Lithium-ion Battery
- Honorary to John B. Goodenough
- What Happens After a Lithium-ion Battery is Connected to a Device?
- Why The Lithium-Ion Battery?
- Why Do Lithium Batteries Explode?
- Battery Manufacturing
- Differences Between Car and Phone Battery Manufacturing?
- Alternatives to Lithium-Ion Batteries
- The Ideal Battery
- Is Lithium Abundant?
- Lithium From Mining to Consumers' Hands
- Lithium Mining
- Lithium Extraction
- Lithium Refinement
- Summers of Obsessions
- Other Random Curiosities About Lithium
- Good Resources
- Questions
- Notes
Curiosity = Love
I have a habit of obsession, but not just any kind; it is the most beautiful one of all: following my curiosity without hesitation. I find this obsession to be similar to love. There’s no rhyme or reason to it; it just happens. Therefore, you can say that following my curiosity is one of my loves. I am reminded of the lines Pablo Neruda wrote, and they capture this feeling perfectly:
I love you without knowing how, or when, or from where,
I love you directly without problems or pride: I love you like this because I don’t know any other way to love, except in this form in which I am not nor are you,
so close that your hand upon my chest is mine,
so close that your eyes close with my dreams.
I follow my curiosity without knowing how, or when, or from where. I simply do.
Just as love can’t be explained, nor can following my curiosity. I see love and following my curiosity as the same force of nature. Both are intrinsic and can’t be explained, but once you feel the magic, you can’t unfeel it again.
Love isn’t a journey, but rather a jointly created painting. You can ask me why I love to follow my curiosity, and I won’t know what to tell you. You can ask me how I knew I needed to follow my curiosity, and my answer will make little sense to you. You can ask me all sorts of questions, and I won’t know. But when I follow my curiosity, it’s an artful odyssey that unfolds, stroke by stroke, to make connections no one has done before and paint a unique painting, and therefore, unique life.
Everyone is obsessed with rational reasons, but that kills the magic of love; that kills the magic of following one’s curiosity. Why love? Why follow your curiosity? If I were telling this to a lover, I would say something about her eyes, her personality, or the way she makes me feel. I could come up with a Million Reasons if Lady Gaga asked me (and baby, "one good one to stay" won't be enough).
But the truth is many people have those qualities, even the most seemingly unique. True love can't be explained because it's like magic. The magic of love is not knowing exactly what is behind it. The magic of love is knowing to follow it without hesitation. Such is the nature of following one’s curiosity.
I don't want to ever have to dissect why I love someone or why I follow my curiosity. If I do, I will come up with these rational reasons which will make me view my loves as commodities and undermine my love for them.
Why love? Why follow my curiosity? I just do. I don't understand, and I don't want to understand because I want my heart to be fully taken by the magic of love, by the magic of following my curiosity.
True love isn't rational and cannot be explained. It's a feeling that goes beyond understanding and reason. People may want to try to dissect love into logical parts, but true love can never be dissected because true love is intrinsic, magical, unexplainable yet present, and in my case, beautifully curious.
How To Read This Book
Read it as an exploration to learn not only about lithium but also about theoretical physics, batteries, manufacturing, history, dangers, isotopes, nuclear energy, refinement, mining, and lithium’s many applications—from drugs and engine greasers to nuclear bombs and lithium-ion batteries.
This book grew out of an obsession. I started with a simple question, "What is Lithium?"
I would ask myself questions and find answers by searching the internet, emailing people, reading books, and trusting that everything would make sense in the end.
My process is simple: I write to teach myself. Writing is a medium of expression that forcefully grabs you by the neck, compelling you to discover what you truly think or know (or don't know). Writing is unforgiving because it exposes your vulnerabilities, leaving you naked in front of your insecurities. At the same time, it's mesmerizing because once you learn to trust yourself, every moment becomes an experience that connects you to the true purpose of the human journey. You realize that you always knew and felt more than you thought. Writing consistently squeezes Déjà vus.
I ask myself questions and find a way to answer them. I've found that writing is an effective way to teach myself.
So, I began with understanding lithium at a fundamental level. I looked up its position on the periodic table, its atomic weight, its atomic number, and everything that followed.
Soon enough, I realized I didn’t know much about theoretical physics. I understood what protons, neutrons, and electrons were, but what are they exactly? This led me to explore isotopes, radioactivity, nuclear energy, fusion, reactors, and how lithium was used in the past.
But then, I had a thought, “What about the history?” I always, always love to learn about the history of whatever I’m learning because it gives me perspective and a less subjective mind frame to understand the topic. I try to put myself in the shoes of the first person who came across lithium and decided to name it “Lithium.”
Or even understanding what lithium actually looks like. Did you know? Lithium is like silver cheese and soft enough you can cut it with a knife.
The process begins, and I start learning wherever my curiosity takes me. This process may seem random, but you quickly realize how much more of the world you notice. One day, I was learning about how lithium was used to treat mania and bipolar disorder. And guess what? On that same day, I overheard someone in a pharmacy asking for "Lithium Carbonate," and that moment transported me into the history of using Lithium Carbonate for these disorders.
Reality becomes deeper, with many layers to appreciate and explore, and along the way, I learn about myself and my place in the world.
As I continued my exploration, I ended up learning about the extraction and refining processes of lithium, as well as its chemical and physical properties. This journey also led me to batteries – how they're made, used, and became a true adventure.
I wrote this book to teach myself, to explore opportunities, and simply because I felt like it.
At times, you may wonder, "Isn't this book about lithium?" Let me reassure you that it is indeed centered around lithium, but I encourage you to trust the journey. By exploring all aspects of lithium, I aim to provide a whole perspective to understand and appreciate it fully. After all, as the saying goes, "No man is an island," and the same applies to lithium—it is intricately connected to a vast array of subjects and knowledge.
Enjoy it as much as I did.
Lithium: An Atom's Perspective
Lithium (Li) is a chemical element, meaning it cannot be broken down into other elements. Beyond lithium, the journey downward into the world of matter hits a limit, with atoms marking the primary exploration point.
But are atoms the end? Or could you keep on going down? Shall we go further into the depths? Heck yeah, why not?
Atoms are the smallest particles that make up everything around us. Think about it like this: atoms like little solar systems with a central part called the nucleus, housing even tinier entities known as protons and neutrons.
But hold on, we're not done yet! Circling around the nucleus, we have electrons, even smaller in comparison. Now, you might think that's the end of the line, but nope, we have more! Protons and neutrons themselves are composed of even tinier particles called quarks. And here's where it gets interesting - some scientists speculate that there could be something smaller than quarks, and they call it prions, though the evidence isn't rock-solid yet.
To give you an idea of scale, the nucleus of an atom is as tiny as a grain of sand. In comparison, the entire atom, including its electron cloud, would stretch to roughly the size of a football stadium.
Now, to grasp the proportions further, let's think about the nucleus in relation to a proton or neutron. It's like comparing that minuscule grain of sand to an even smaller particle within it, say, a speck of dust.
What about quarks? They’re mysterious point-like particles, meaning they have no known size or substructure but I’ll still give you an analogy anyways. If we were to compare the size of a quark to the nucleus of an atom, it would be an incredibly tiny particle hiding within that grain of sand.
Keep in mind that these comparisons are mere approximations. The true scale of these subatomic entities is nearly impossible for human comprehension. Plus, sizes might vary depending on the context and experimental observations, so take it all with a grain of quark!!
Earlier, I mentioned that lithium is a chemical element, and we have already learned what that means. If you look it up in the periodic table, you'll find it with the number 3 associated with it. This number is unique to each element and represents the count of protons present in the nucleus of an atom of that element. So, for lithium, there are three protons within its nucleus.
But what do 'protons present in the nucleus of an atom of that element' mean? Well, every atom has a central part called the nucleus, which you can think of as the party's epicenter. In this nucleus, you'll find protons and neutrons mingling together. And just nearby, surrounding the nucleus, are the electrons, adding to the lively atmosphere.
So, protons have a positive electrical charge, while neutrons remain neutral with no electrical charge at all. This distinction will be crucial in a moment so keep that thought.
Lithium, holding an atomic number of 3, means that every single lithium atom has three protons residing in its nucleus. So, if you were to peek inside the nucleus of a lithium atom, you'd discover those three protons, each carrying a positive charge.
Now, what else is within a lithium atom? Take a guess!
Exactly! Neutrons and electrons!
Neutrons are particles found in the nucleus that bear no electric charge; they are entirely neutral. Interestingly, their mass is just a bit larger than that of protons. As for most lithium atoms, you'll typically find around 4 or 5 neutrons, contributing to lithium's atomic mass (Atomic mass = number of protons + number of neutrons + number of electrons). However, the exact count of neutrons can vary, leading to different isotopes of lithium, each with its unique atomic mass.
Isotopes? What, in the world, is that?
Isotopes: Lithium's Siblings
What are isotopes, you ask? Isotopes are like siblings. They share the same parents (meaning they have the same number of protons), but here's the twist: they can have different numbers of neutrons (kind of like varying body mass in siblings). So, just like siblings, isotopes can exhibit similarities, but they can also display distinct characteristics.
What do you call an isotope that's always getting into trouble? A radioactive sibling!
That was hilarious. If you didn’t laugh, please laugh.
Moving on, let's explore the specific isotopes of lithium. Among them, two stand out as the well-behaved ones (they’re stable): Lithium-6 and Lithium-7.
Lithium-6 comprises 3 protons and 3 neutrons, whereas lithium-7 contains 3 protons and 4 neutrons. It's important to note that these isotopes occur naturally, meaning they exist in nature without any human intervention. In other words, they are naturally existing forms of the element. Among the various isotopes, lithium-7 takes the lead as the most abundant isotope of lithium.
Lithium-6 and lithium-7 have a cool quality - they are stable isotopes, meaning their atomic nuclei are balanced and naturally resistant to breaking down or decaying over time. These stable isotopes have been in existence for billions of years and are expected to maintain their stability for an incredibly long time, even surpassing the age of the universe! When we talk about stability in this context, it refers to the nucleus of the isotope remaining intact, without undergoing radioactive decay to transform into other elements or isotopes.
Nevertheless, stable isotopes like lithium-6 and lithium-7 can still take part in chemical reactions and physical processes. So, while their nuclei remain unchanged, interactions and changes can occur at the chemical or physical level.
In addition to stable isotopes, we also have radioactive or unstable isotopes. These isotopes have nuclei that are inherently unstable, leading to spontaneous radioactive decay. This decay involves the release of radiation in the form of particles or electromagnetic waves, as the nucleus undergoes transformation to reach a more stable configuration. The process of radioactive decay is entirely random and unpredictable, and it continues until the isotope eventually reaches a stable state.
The instability of these isotopes arises from an imbalance of protons and neutrons in their atomic nuclei. Think of it like a group of people trying to balance on a seesaw. If the group has an equal number of people on each side of the seesaw, it remains stable and won't tip over. However, if there are more people on one side than the other, the seesaw becomes unstable and will eventually tip over. Unstable isotopes are similar to that group on the seesaw that's out of balance. The imbalance of protons and neutrons in the nucleus of an unstable isotope is what causes its inherent instability and eventual decay.
As a result, unstable isotopes demonstrate radioactive properties and ultimately transform into other isotopes or elements over time. For instance, let's consider lithium-8 (3 protons and 5 neutrons), an example of a radioactive isotope. Due to its unstable nucleus, lithium-8 undergoes radioactive decay, transitioning into a different element. The decay process in this case is called beta decay, where one of the neutrons in the nucleus transforms into a proton, emitting an electron (beta particle) in the process.
Consequently, the resulting nucleus has one additional proton, and as you know, the number of protons determines the type of element. Since lithium has three protons, the decay of lithium-8 results in the nucleus gaining one more proton, effectively transforming it into beryllium-8 (Be-8) through beta decay.
The decay process of lithium-8 in chemistry language:
Lithium-8 (Li-8) → Beryllium-8 (Be-8) + electron (β-)
Now, here's where it gets even more interesting. Beryllium-8 is also an unstable isotope and experiences rapid decay (within fractions of a second) to achieve a more stable configuration. What does it decay into? Well, that depends on specific circumstances and energy levels, but under certain conditions, beryllium-8 can decay into two helium-4 nuclei, leading to the formation of two separate helium atoms.
The decay process of beryllium-8 in chemistry language can be represented as:
Beryllium-8 (Be-8) → Two Helium-4 nuclei (2 He-4)
After beryllium-8 (Be-8) transforms into two helium-4 nuclei (2 He-4), the story doesn't end there. These two helium-4 nuclei can continue to undergo various nuclear reactions and decay depending on the specific circumstances and energy levels involved.
The fusion of the two helium-4 nuclei is an intriguing possibility, leading to the creation of a heavier nucleus. This remarkable process, known as helium fusion, plays a pivotal role in stellar nucleosynthesis, where stars produce and synthesize elements. Within the intense heat and pressure of stars, helium fusion facilitates the formation of heavier elements like carbon, oxygen, and beyond.
In the context of stellar nucleosynthesis, the decay of beryllium-8 holds immense importance. It serves as the initial step in the triple-alpha process, which is the mechanism by which stars generate carbon. In the triple-alpha process, three helium-4 nuclei come together, amalgamating to form carbon-12. The decay of beryllium-8 is a vital component for this process to take place, providing the necessary energy to enable the combination of three helium-4 nuclei.
The nucleus of a lithium atom is similar to a tightrope walk, delicately teetering on the edge of instability. It possesses a unique nature that sets it apart from other elements. Remarkably, the stable isotopes of lithium found in nature exhibit some of the lowest binding energies per nucleon among all stable nuclides.
In simpler terms, the atomic nucleus of lithium is not as tightly bound compared to other elements. This property is essential because it makes lithium a highly reactive element. Its loosely bound third electron can be easily lost, enabling lithium to form bonds with other elements. This reactivity is why lithium is commonly used in batteries. Additionally, the loosely bound third electron facilitates the fusion of lithium nuclei with other nuclei, making lithium a promising fuel for potential future fusion reactors.
Due to its distinct nuclear characteristics, lithium is not as abundant in the solar system as one might expect. Surprisingly, it is less common than 25 of the first 32 chemical elements, despite having lightweight nuclei. This intriguing fact makes it an exception to the usual trend where heavier nuclei are generally less common.
However, don't let its relative scarcity deceive you. Lithium holds immense significance in nuclear physics and has crucial applications in this field. A groundbreaking achievement occurred in 1932 when scientists successfully transmuted lithium atoms into helium through what became the first human-made nuclear reaction. This pivotal milestone represented a remarkable leap forward in our comprehension of nuclear processes.
Moreover, the compound lithium deuteride, formed from lithium and deuterium (a heavy isotope of hydrogen), possesses incredible power as a fusion fuel. In staged thermonuclear weapons, this potent combination triggers a controlled fusion reaction, unleashing a tremendous amount of energy. It’s similar to a controlled cosmic explosion, harnessed through human ingenuity.
The distinct nuclear characteristics of lithium make it a captivating element for scientific investigation and application in the domain of nuclear physics. Its contribution to advancing our comprehension of the atomic world and its potential for generating formidable energy underscores its importance in this wynorrific field.
"I'm radioactive, radioactive": The Magic of Radioactive Decay and the Charms of Radioactivity
You may wonder, "What exactly happens for an isotope to undergo radioactive decay and become stable?" Radioactivity is the spontaneous emission of radiation from the nucleus of an atom, a natural property of radioactive isotopes. Radioactive decay typically [1] occurs when the forces within the atomic nucleus are unbalanced due to an excess of protons or neutrons. Various types of radioactive decay exist, including alpha decay, beta decay, and gamma decay.
- Alpha decay: The nucleus emits an alpha particle, consisting of two protons and two neutrons (equivalent to a helium nucleus). This emission reduces the atomic number by two and the mass number by four.
- Beta decay: The emission of a beta particle, which can be either an electron or a positron. During this process, a neutron or proton within the nucleus undergoes a transformation. Beta decay results in a change of the atomic number by one while preserving the total mass number. There are two types of beta decay:
- Beta-minus (β-) Decay: A neutron is converted into a proton, and an electron (beta particle) and an antineutrino are emitted.
- Beta-plus (β+) Decay: A proton is converted into a neutron, and a positron (positively charged beta particle) and a neutrino are emitted. [2]
- Gamma decay: An excited nucleus transitions from a higher energy state into a lower energy state, releasing a gamma ray photon. Unlike alpha and beta decay, gamma rays do not alter the atomic number or mass number of the nucleus; they release energy without changing the nucleus composition. Gamma rays are often emitted alongside alpha or beta particles during radioactive decay and possess the highest penetration power among the emitted radiations.
Apart from the mentioned decay processes, there are two additional mechanisms:
- Positron Emission: A proton is converted into a neutron, and a positron (positively charged electron) and a neutrino are emitted.
- Electron Capture: A proton combines with an inner-shell electron, converting the proton into a neutron and emitting a neutrino. [3]
Radioactive decay is a random process, making it impossible to predict the timing of individual decay. However, when we consider a large number of radioactive atoms, their decay follows a statistically predictable pattern described by the concept of half-life. The half-life represents the time it takes for exactly half of the radioactive atoms in a sample to undergo decay.
It's crucial to note that the definition of half-life for discrete entities, like radioactive atoms, is grounded in probability rather than a literal "half" remaining. Specifically, the half-life is defined as the time required for exactly half of the entities to decay on average. In other words, the probability of a radioactive atom decaying within its half-life is 50%. For instance, in the simulation of the decay of many identical atoms, we observe that after one half-life, there are not precisely one-half of the atoms remaining, but approximately so due to random variation in the process. Nonetheless, the law of large numbers suggests that with a significant number of identical atoms decaying, it becomes an excellent approximation to say that half of the atoms remain after one half-life.
For instance, if a certain radioactive isotope has a half-life of 1 hour, it signifies that after 1 hour, half of the initial number of radioactive atoms will have decayed.
Why are individual decays unpredictable? It's due to the seemingly random nature of quantum mechanics, which governs the behavior of particles at the atomic and subatomic levels.
Each radioactive has a certain probability of decaying, but this probably cannot be determined for any individual atom. This is the same thing as predicting a single coin toss with certainty; it's simply not feasible. However, we can make statistical predictions based on a large number of coin tosses.
Fortunately, mathematics offers us insight into this mystery of randomness. Enter the law of large numbers! This law states that as the number of trials or observations increases, the average of those trials or observations will converge to the expected value or true probability. In the context of radioactive decay, this means that although we cannot predict when an individual atom will decay when we have a large number of atoms, the average rate of decay over time becomes predictable.
Let’s use an example. Consider a sample of 1000 radioactive atoms with a half-life of 1 hour. After 1 hour, on average, we would expect half of the atoms, which is 500, to have decayed. After another hour, half of the remaining 500 atoms will decay, leaving us with 250 atoms. This pattern continues, and the number of radioactive atoms decreases by half in each successive time interval equal to the half-life. While we can't predict which atoms will decay at any given time, we employ the power of statistical probabilities to comprehend the overall behavior of a large number of atoms.
As we untangle the world of radioactive decay, we learn not only the intricacies of quantum mechanics governing this process but also the statistical predictability hidden in large numbers. However, our journey into radioactivity does not end here; it takes us beyond decay to explore the vast collection of applications that have revolutionized our world.
Radioactive materials and their decay processes have found invaluable use cases in scientific research, medical diagnostics, cancer treatments, energy production, and even archaeological dating, leaving a mark on each domain.
Yet, as we continue moving along, we must navigate through misconceptions surrounding radioactivity and acknowledge potential dangers, highlighting the significance of responsible practices in handling radioactive materials to ensure safety and mitigate risks.
Applications of Radioactivity
First and foremost, let's explore the use cases of radioactivity, exploring each application one by one:
-
Radiometric Dating: This technique allows scientists to determine the age of geological formations, archaeological artifacts, and fossils. By measuring the ratio of original radioactive isotopes (parent isotopes) to newly formed isotopes (daughter isotopes) and utilizing the decay rate, one can calculate the time that has elapsed since the material was formed.
-
Nuclear Power: Nuclear power plants use controlled nuclear reactions to produce electricity. The process involves the controlled release of energy through the radioactive decay of certain isotopes, such as uranium-235 or plutonium-239.
-
Medical Imaging and Radiotherapy: Radioactive isotopes find essential roles in medical imaging techniques like positron emission tomography (PET) and single-photon emission computed tomography (SPECT). These methods aid in diagnosing and monitoring diseases by detecting the distribution of radioactive traces within the body. Similarly, radiotherapy employs controlled radiation to target and destroy cancer cells, offering a vital treatment option.
-
Industrial Applications: Radioactive isotopes are widely used in industrial settings, facilitating ultrasonic thickness gauging in manufacturing processes, quality control in material testing, and flow rate measurements in pipelines.
-
Food Preservation: The process of irradiation, which involves exposing food to controlled levels of radiation, extends shelf life and prevents spoilage by effectively eliminating bacteria, insects, and other pests. This technique is particularly useful in preserving fruits, vegetables, meat, and spices, and it is approved by the FDA for specific applications.
-
Smoke Detectors: Ionization smoke detectors employ a small amount of radioactive material, typically Americium-241, to detect smoke particles in the air. When smoke enters the detector, it disrupts the ionization process, triggering an alarm and alerting occupants to potential fires.
-
Sterilization of Medical Equipment: Radiation is utilized to sterilize medical equipment, damaging the DNA of microorganisms and effectively eliminating them through the use of gamma rays and electron beams.
-
Geological Surveys and Explorations: Gamma-emitting isotopes assist in studying the composition of rocks, minerals, and soil, as well as in prospecting for oil and gas.
-
Environmental Monitoring: Radioactive tracers, such as isotopes of radon or cesium, play a crucial role in tracking the movement of air, water, and pollutants in the environment.
-
Scientific Research: Radioactive isotopes serve as powerful tools in scientific research across various disciplines, including genetics, molecular biology, nuclear physics, and environmental sciences.
These are just a few uses of radioactive isotopes that help make our world as we know it today. However, with such fascinating uses, it's only natural to wonder about safety concerns. You might be thinking, 'Excuse me, is this safe?'"
I’ll spoil it for you: It is safe. But like any powerful tool, improper handling can lead to potential harm.
Let's take a moment to examine both the common misconceptions surrounding radioactivity and the potential dangers it has.
Misconceptions: Is The Use of Radiation Safe?
Misconception: All types of radiation are harmful.
While it is true that high levels of certain types of radiation, such as ionizing radiation, can be harmful to living organisms, not all forms of radiation pose significant risks. For instance, non-ionizing radiation, like visible light or radio waves, is generally considered safe at typical exposure levels and does not present the same dangers as ionizing radiation.
Misconception: Any amount of radiation exposure is dangerous.
The potential harm from radiation exposure depends on the dose received. Low levels of radiation, such as those encountered in everyday life from natural sources (the sun, etc.), are generally considered safe and unlikely to cause immediate harm. Radiation exposure becomes a concern at higher doses or prolonged exposure, where the cumulative effect can lead to adverse health effects.
Misconception: Radiation from medical imaging is always harmful.
Medical imaging techniques, such as X-rays or CT scans, use ionizing radiation to create diagnostic images. While it is true that repeated or excessive exposure to medical radiation can pose risks, the benefits of these diagnostic tools often outweigh the potential harm. Certain everyday activities, such as flying in airplanes, living at higher altitudes, or undergoing certain medical procedures, may lead to higher radiation exposure, but the associated risks are generally considered minimal when compared to other risks we willingly accept, like accidents, exposure to environmental pollutants, or unhealthy dietary habits. Although the argument for comparative risks may not be convincing to everyone, it emphasizes the importance of keeping radiation exposures well below established safety limits.
In the case of healthcare, medical professionals follow strict guidelines to ensure radiation doses are kept as low as reasonably achievable while providing necessary diagnostic information and treatment. We'll talk about this topic further in a bit.
Misconception: Radiation is always cumulative in the body.
The human body has natural mechanisms to repair and eliminate damage caused by low levels of radiation exposure. For most people, the body can effectively deal with small amounts of radiation encountered in daily life without significant long-term accumulation. However, chronic or repeated exposure to higher levels of radiation can overwhelm these protective mechanisms, leading to potential health risks.
Misconception: Radiation shielding and safety measures are unnecessary because radiation exposure is always dangerous.
While it is true that high levels of radiation exposure can be harmful, proper shielding and safety measures are essential in environments where radioactive materials are present to minimize potential risks. These measures are not meant to create unnecessary fear but to ensure the safety of workers and the public. By wearing protective clothing, using shielding materials, and following safety protocols, the risk of radiation exposure can be significantly reduced. These measures are based on scientific research and guidelines from regulatory agencies to ensure that radiation doses remain within acceptable limits and do not pose immediate health hazards.
Misconception: Radioactive waste management is not a significant concern because the waste will eventually become harmless.
It is a misconception to assume that radioactive waste will naturally become harmless over time. Radioactive materials can remain hazardous for extended periods, and improper waste management can lead to serious environmental contamination and potential health hazards. Proper storage, containment, and disposal methods are crucial to prevent the release of radioactive substances into the environment. Careful management ensures that the waste is stored securely and monitored to prevent any potential leaks or breaches. By following rigorous waste management protocols, the long-term safety of both the environment and human health can be safeguarded.
Misconception: Nuclear energy is bad. For example, look at countries like Germany shutting down their nuclear plants.
In three polite words: Germany was stupid. Germany's decision wasn't based on nuclear power as an energy source but rather on political and public sentiments. Nuclear energy, when managed responsibly, has numerous benefits. It is a low-carbon energy source, contributing significantly to greenhouse gas emission reduction and combating climate change. Moreover, nuclear power plants can provide a stable and reliable energy supply, which is essential for countries seeking energy security and independence. Many countries continue to embrace nuclear energy as part of their energy mix to achieve sustainable and cleaner energy production. Rather than dismissing nuclear energy outright based on isolated incidents, a balanced and informed assessment of its benefits and drawbacks is crucial for shaping the future of global energy systems.
Misconception: Nuclear accidents are too rare to warrant stringent safety measures and emergency preparedness.
While nuclear accidents are indeed rare, their potential consequences are severe and far-reaching. These incidents highlight the importance of stringent safety measures, emergency preparedness, and effective response protocols. It is crucial to have comprehensive plans in place to minimize the impact of nuclear accidents and protect the public. Even though the probability of an accident may be low, the potential consequences are significant enough to warrant robust safety measures. By being prepared and proactive, we can mitigate the risks associated with nuclear accidents and ensure a swift and effective response if such an unfortunate event were to occur.
So, is radioactivity dangerous? High levels of radiation exposure can indeed have serious health consequences, including an increased risk of cancer, tissue damage, and genetic mutations. Accidental exposure to high doses of radiation, such as in nuclear accidents, can lead to acute radiation sickness
The next time you encounter the term "radiation," it's essential not to jump to conclusions or panic. Instead of accepting everything at face value, take a moment to consider important factors like the type of radiation, the dose level, and the specific element being used. Understand the science behind it, understand how everything works, and ask relevant questions. Be cautious about blindly trusting regulators or so-called "experts" without seeking further knowledge and understanding, not only when it comes to radiation but in any aspect of life.
Radiation in the United States
I must admit the higher radiation exposure in the United States compared to other countries is unsettling. Radiation exposure is a real head-scratcher, leaving us with more questions than answers about how it affects our health.
The United States, in particular, has seen a significant rise in total per capita radiation exposure, nearly doubling since the 1980s.
The average American gets rewarded with about 620 millirems (0.62 rem) of radiation every year. Half of that comes from natural background radiation, like radon gas seeping into homes from soil and rocks. And guess what? Radon and thoron gases make up two-thirds of this exposure, with the rest coming from cosmic rays and the Earth itself.
The thing is, these "background doses" vary depending on where you live. Living in Denver? You'll be getting slightly higher radiation exposure. The atmosphere is thinner at higher altitudes, so there is less protection from cosmic radiation. This means that people who live at higher altitudes are exposed to more cosmic radiation.
But wait, there's more! The big spike in total radiation exposure is mainly because of the boom in medical imaging. And you know what? Medical imaging accounts for about 96% of human exposure to man-made radiation. Yikes!
Sure, procedures like chest X-rays and CT scans are lifesavers, giving doctors critical diagnostic info. But a chest X-ray can give you about 0.1 millisieverts (mSv) of radiation, while a whole-body CT scan slaps you with around 10 mSv. Just to put things in perspective, the average annual background radiation level in the US is around 3 mSv. So, yeah, that CT scan could be hitting you with a bunch more radiation than you'd usually get in a whole year.
Look, medical imaging is crucial for timely and accurate diagnoses, and it saves lives. But we can’t forget its risks. Using these imaging techniques carefully and following strict guidelines can minimize radiation exposure while still giving doctors the information they need to work their magic. So, it's a balancing act between the benefits and risks, but being informed and making thoughtful decisions is the key while continuing to find ways to reduce radiation with innovation.
I’ll close this topic with some facts to think about:
- The average annual background radiation level in the United States is about 3 millisieverts (mSv)
- A chest X-ray exposes a person to about 0.1 mSv
- A whole-body computerized tomography (CT) scan exposes a person to about 10 mSv
- Medical procedures account for nearly all (96%) human exposure to man-made radiation
- Most of the increased exposure in the United States is due to CT scanning and nuclear imaging, which require larger radiation doses than traditional x-rays
- The effective doses from diagnostic CT procedures are typically estimated to be in the range of 1 to 10 mSv
- The amount of radiation from one adult chest x-ray (0.1 mSv) is about the same as 10 days of natural background radiation that we are all exposed to as part of our daily living
- The US National Council on Radiation Protection and Measurement reported that medical x-rays and nuclear medicine account for 15% of all radiation exposures
Here are the sources if you want to look further:
-
Radiation risk from medical imaging - Harvard Health Publishing.
-
Radiation Dose - RadiologyInfo.
-
Radiation Exposure of medical Imaging - National Center for Biotechnology Information.
-
What are the Radiation Risks from CT? - U.S. Food and Drug Administration.
-
Background Radiation - U.S. Environmental Protection Agency.
-
Frequently Asked Questions (FAQ) About Radiation Protection - U.S. Nuclear Regulatory Commission.
-
Doses in Our Daily Lives - US.NRC.
-
Awareness of ionizing radiation and its effects among clinicians - Reddy Ravikanth.
A Journey to the Subatomic World: Theoretical Physics, Particles, and Matryoshkas
Now that we have explored the intriguing world of isotopes and their radioactive decay, it's time to get a journey to the center of the earth, I mean the universe.
In our earlier discussion, we explored the concept of radioactive decay, specifically the transformation of a neutron into a proton. Now, armed with the appropriate terminology, we can describe this phenomenon as beta decay.
But before we get into beta decay, I want to make a brief comment to understand the significance of theoretical physics and why you should care about it.
Theoretical Physics: The Secret to Your Lover's Heart
Imagine you have stumbled upon a magical treasure chest that reveals the secrets of the universe. As you open it, you not only discover the wonders of lithium but also the captivating world of theoretical physics.
Initially, this might seem like an unrelated detour, but let me explain why it's worth your attention. Understanding theoretical physics reveals the fundamental principles that govern the behavior of matter and energy, offering a profound understanding of the underlying forces that shape our world. It's like peeking into the gears of the machinery of the universe, where planets, stars, and galaxies alongside quarks, particles, and bosons dance to orchestrate the intricate symphony of the universe.
Theoretical physics embodies the essence of pure human curiosity to discover the secrets of the universe. It's like a set of Matryoshka dolls, each layer revealing more profound insights, and embodies the nature of scientific reductionism—breaking down complex phenomena into fundamental principles. Moreover, it offers the thrill of a Steve Jobs' "One More Thing..." moment, always surprising us with new revelations. The allure of theoretical physics lies in its boundless potential, as it seems to have no end, inviting us to venture into the unknown and explore the infinite wonders of the cosmos.
Theoretical physics might sound like all theoretical mumbo-jumbo, but trust me, it's more exciting than you think! So, why is it called "theoretical" physics? I’ll give you an analogy.
You know how when you're into someone, you start theorizing about your feelings and whether they feel the same way? Those theories you come up with aren't fake or meaningless; they actually help you understand your emotions and guide your actions. Well, in theoretical physics, it's the same thing. The term "theoretical" doesn't mean it's all fake or irrelevant (unless you’re talking about string theory 😉). It's more about the process of creating theories and mathematical models to figure out how the physical world works.
Just like your love theories help you navigate relationships, theoretical physicists use their theories to tackle the mysteries of the universe. They come up with wild ideas, use fancy math equations, and use logical thinking to build these mind-blowing theoretical frameworks that explain natural phenomena. And here's the cool part – these theories aren't just wild guesses; they're backed by real observations, experimental data, and the core principles of physics.
So, just like your understanding of feelings guides your actions, theoretical physics guides experimental physicists. It helps them design experiments and make sense of their results. With these theoretical frameworks, scientists can predict and explain how particles behave, how celestial bodies move, and how the fundamental forces shape our entire universe.
Don't let the word "theoretical" fool you! Theoretical physics is the path to untangling the secrets of the cosmos, and it's way more fascinating than you might've thought! And when it comes to love and physics, theories are the driving force - whether you're searching for the mysteries of the universe or trying to decode your crush's feelings! Keep theorizing, and who knows, maybe one day you'll have your own "Eureka!" moment in both love and physics!
Time to talk about theoretical physics and beta decay.
From Beta Decay to Bosons and Fermions
Beta decay is a fascinating transformation where a neutron can turn into a proton or vice versa, involving the emission of an electron (β-) or a positron (β+) and a neutrino or an antineutrino. It's a fundamental process that plays a crucial role in the stability of atomic nuclei.
Now, to understand beta decay better, we need to uncover the hidden players in this subatomic dance - the quarks. Quarks are the tiny building blocks that make up protons and neutrons, and they reside within the nucleus of an atom. Both protons and neutrons consist of three quarks each, forming a dynamic trio that gives these particles their unique properties.
Surprisingly, quarks are elusive creatures; they are never found alone and are always paired together in groups of three.
Neutrons consist of three quarks. Two of these quarks are down quarks, which carry a negative electric charge, while the third quark is an up quark, possessing a positive electric charge. Now, you might wonder, how can this combination result in a neutral overall charge for the neutron? It might seem puzzling, but fear not – physicists have a clever trick up their sleeves!
Let's break it down. Each down quark has a charge of -⅓, and the lone up quark has a charge of +⅔. When we add them together, -⅓ + (-⅓) + (+⅔) equals 0. Voilà! A NEUTRAL CHARGE is achieved. Neutrons would be great diplomats.
What about protons? Protons are composed of three quarks: two up quarks, each carrying a charge of +⅔, and one down quark, which has a charge of -⅓. Adding up the charges of these quarks, we get:
(+⅔) + (+⅔) + (-⅓) = +1
Now that we know what happens under the hook. Let’s take a closer look at the world of beta decay.
This intriguing process involves the transformation of a proton into a neutron (Beta-plus (β+) decay) or a neutron into a proton (Beta-minus (β-) decay).
But wait, you might wonder, how do these quarks change their charges during beta decay? Well, it's not a spontaneous occurrence; there's an interesting interplay of particles involved.
During beta-minus decay, a negatively charged W- boson enters the scene and participates in the conversion process. It helps the down quark inside the neutron change its charge from negative (-1/3) to positive (+2/3). As this transformation unfolds, an electron/beta particle (β-) is emitted from the nucleus to conserve the electric charge.
On the other hand, in beta-plus decay, a positively charged W+ boson takes center stage when an up quark inside the proton transforms into a down quark. This change in the quark's charge from positive (+2/3) to negative (-1/3) is made possible by the W+ boson's involvement.
What are these W bosons all about? W bosons are elementary particles, which means they are subatomic particles that exist on a much smaller scale than an atom and are not made up of smaller particles themselves. W bosons play a crucial role in nuclear decay, like beta decay in radioactive elements. Additionally, they are responsible for creating heavier elements, such as helium and lithium, through fusion in stars' cores.
The weak force, mediated by the W bosons, has been instrumental in the evolution of the universe, allowing the formation of vital elements like carbon and oxygen, which are essential for life.
As you've seen, there are two types of W bosons: the W+ and the W-. These bosons play a crucial role in interactions and transformations involving elementary particles, like what we observed in beta decay.
The significance of W bosons goes beyond their names. They are responsible for mediating the weak nuclear force, which is one of the fundamental forces of nature. Alongside gravity, electromagnetism, and the strong force, the weak nuclear force plays a fundamental role in shaping the universe.
In the world of particle physics, bosons act as carriers or vehicles for fundamental forces and fields. For example, photons are associated with electromagnetic force, while gluons are linked to the strong nuclear force. Theoretical gravitons, which are yet to be observed, are believed to be associated with gravitational force. And let's not forget the famous Higgs boson, which is linked to the Higgs field and is responsible for giving mass to other elementary particles.
W bosons are not the only ones that carry the weak nuclear force. We can’t forget his cousin: The Z boson. This little guy is a neutral elementary particle, meaning it has no electric charge. Just like its charged cousin, the W boson, the Z boson is in charge of the weak force.
Now, the Z boson may be electrically neutral, but that doesn't make it any less important. It actually plays a similar role to the W bosons in handling the weak force. The cool thing is that the Z boson can only interact with particles that have no charge, like those sneaky neutrons.
This Z boson has a few essential jobs in the universe. First, it's the mastermind behind neutrino scattering. Neutrinos are these elusive particles, and the Z boson helps them bounce off matter, which scientists use to study neutrino properties.
Next, the Z boson knows how to handle unstable particles, like the mysterious tau lepton. It can step in and help these particles undergo their special kind of decay.
But that’s not all! The Z boson is like a magician; it can create antimatter, the opposite of regular matter. Antimatter is super rare in the universe, but scientists can actually make it using particle accelerators.
I mentioned W and Z bosons. What is a…boson? And where does that name come from?
A boson is a type of elementary particle that belongs to a class of particles called force carriers. Bosons are responsible for mediating fundamental forces between other particles. The name "boson" comes from the Indian physicist Satyendra Nath Bose, who collaborated with Albert Einstein in the 1920s to develop a statistical model for particles with integer spin. The model, now known as Bose-Einstein statistics, describes the behavior of bosons at very low temperatures.
Bose was born in Kolkata, India in 1894. He studied mathematics and physics at the University of Calcutta, and then went on to study at Cambridge University in England. In 1924, he published a paper in which he proposed a new statistical model for particles with integer spin. The model was based on the idea that these particles could occupy the same quantum state.
In this model, particles could either have integer values of spin (0, 1, 2, etc.), in which case they are called bosons, or half-integer values of spin (1/2, 3/2, 5/2, etc.), in which case they are called fermions.
Dirac was impressed with Bose's work, and he coined the name "boson" to honor him. Dirac also showed that Bose-Einstein statistics could be used to explain the behavior of photons, which are particles of light. Photons are examples of bosons.
Bosons are fascinating because they behave quite differently from fermions. For example, multiple bosons can occupy the same quantum state, leading to phenomena like Bose-Einstein condensation, where a large number of bosons all occupy the same low-energy state and behave as a single quantum entity. This behavior is crucial in understanding the properties of certain materials, such as superfluids and superconductors.
Bose's work has had a profound impact on our understanding of the the universe. Bose-Einstein statistics are used to describe the behavior of a wide variety of particles, including photons, gluons, and the Higgs boson. It is also used to explain the behavior of superfluids and Bose-Einstein condensates.
If you find this specific topic fascinating, you’ll truly enjoy my conversation with Tony Leggett who was awarded the Nobel Prize in Physics for his work on superfluidity and Bose-Einstein condensation.
Leggett's work has helped to explain the behavior of these exotic states of matter, which are characterized by the macroscopic quantum behavior of bosons.
One of Leggett's most important contributions was the development of a theory of superfluidity in liquid helium-3. He showed that liquid helium-3 could exist in two different phases: a normal phase and a superfluid phase. The superfluid phase is characterized by the absence of friction, which allows the liquid to flow without any resistance.
Leggett also developed a theory of Bose-Einstein condensation in dilute gases. He showed that a dilute gas of bosons could become a Bose-Einstein condensate if it was cooled to a sufficiently low temperature. A Bose-Einstein condensate is a state of matter in which all of the bosons in a system occupy the same quantum state.
Leggett's work on superfluidity and Bose-Einstein condensation has had a profound impact on our understanding of these exotic states of matter. His work has also led to the development of new technologies, such as quantum computers and sensors.
You can watch my conversation with Nobel laureate Tony Leggett, here.
Bosons and there’s something else called fermions.
All of the particles in the universe can be classified into two types: bosons and fermions. Bosons have an integer spin, while fermions have a half-integer spin. Bosons are responsible for mediating the four fundamental forces of nature, while fermions make up all of the matter in the universe.
Fermions obey the Pauli exclusion principle, which states that no two fermions can occupy the same quantum state. This principle is responsible for the structure of matter, as it prevents electrons from collapsing into the nucleus of an atom.
The name "fermion" was coined by Paul Dirac in honor of Enrico Fermi, an Italian physicist who made significant contributions to the development of quantum mechanics and particle physics.
Here are some of the most important fermions:
- Quarks are the building blocks of protons and neutrons. There are six types of quarks: up, down, strange, charm, top, and bottom.
- Leptons are fundamental particles that do not interact with the strong force. There are six types of leptons: electron, muon, tau, electron neutrino, muon neutrino, and tau neutrino.
- Neutrinos are very light, neutral particles that interact very weakly with other particles. They are thought to make up most of the dark matter in the universe.
Fermions are essential for our understanding of the universe. They make up all of the matter in the universe, and they play a fundamental role in the structure of matter.
I don’t know if you noticed but Dirac names particles like nobody’s business so who is this Dirac guy?
On Dirac: Naming Monopoly, One Word Per Hour, and Potatoes
Paul Dirac, born in 1902 in England, was a true prodigy. His brilliance led him to St. John's College, Cambridge, where he pursued mathematics in 1921. But you know what? Physics had a different plan for him, and he quickly got hooked on quantum mechanics—a cutting-edge theory of matter and energy being explored at the time.
Then, boom! In 1925, Dirac dropped a paper that shook the world of quantum mechanics. He cooked up a relativistic equation for the electron, that tiny particle that powers electricity. And guess what? His equation went beyond predictions; it revealed the existence of antimatter, the opposite of regular matter. Mind-blowing stuff! Antimatter is super rare in the universe, but we've managed to create a bit of it in particle accelerators.
Oh, but Dirac didn't stop there. He kept slinging groundbreaking contributions to quantum mechanics and particle physics. The cherry on top? In 1933, the Nobel Prize in Physics landed in his hands like a well-deserved gift basket. Why? For his mind-bending work on quantum mechanics, of course. But wait, there's more! Dirac also played a huge part in crafting quantum electrodynamics—a theory that explains the fascinating dance between light and matter.
Dirac was no ordinary physicist; he was one of the greatest minds to grace our scientific universe. But here's the kicker: he was also one of the most private folks you'd ever meet. He shied away from the limelight and rarely sought out publicity. In fact, his colleagues had a cheeky way of measuring shyness—they used a "Dirac" as a unit. One Dirac meant speaking only one word per hour. Now, that's what I call reserved!
If you think I’m joking, this is the transcript of an interview with Dirac where you can how “descriptive” he was with his words:
I been hearing about a fellow they have up at the U. this spring --- a mathematical physicist, or something, they call him --- who is pushing Sir Isaac Newton, Einstein and all the others off the front page. So I thought I better go up and interview him for the benefit of State Journal readers, same as I do all other top notchers. His name is Dirac and he is an Englishman. He has been giving lectures for the intelligentsia of math and physics departments --- and a few other guys who got in by mistake.
So the other afternoon I knocks at the door of Dr. Dirac's office in Sterling Hall and a pleasant voice says "Come in." And I want to say here and now that this sentence "come in" was about the longest one emitted by the doctor during our interview. He sure is all for efficiency in conversation. It suits me. I hate a talkative guy. I found the doctor a tall youngish-looking man, and the minute I seen the twinkle in his eye I knew I was going to like him. His friends at the U. say he is a real fellow too and a good company on a hike --- if you can keep him in sight, that is.
The thing that hit me in the eye about him was that he did not seem to be at all busy. Why if I went to interview an American scientist of his class --- supposing I could find one --- I would have to stick around an hour first. Then he would blow in carrying a big briefcase, and while he talked he would be pulling lecture notes, proof, reprints, books, manuscript, or what have you out of his bag. But Dirac is different. He seems to have all the time there is in the world and his heaviest work is looking out the window. If he is a typical Englishman it's me for England on my next vacation!
Then we sat down and the interview began.
"Professor," says I, "I notice you have quite a few letters in front of your last name. Do they stand for anything in particular?"
"No," says he.
"You mean I can write my own ticket?"
"Yes," says he.
"Will it be all right if I say that P.A.M. stands for Poincare' Aloysius Mussolini?"
"Yes," says he.
"Fine," says I, "We are getting along great! Now doctor will you give me in a few words the low-down on all your investigations?"
"No," says he.
"Good," says I. "Will it be all right if I put it this way --- `Professor Dirac solves all the problems of mathematical physics, but is unable to find a better way of figuring out Babe Ruth's batting average'?"
"Yes," says he.
"What do you like best in America?", says I.
"Potatoes," says he.
"Same here," says I. "What is your favorite sport?"
"Chinese chess," says he.
That knocked me cold! It was sure a new one on me! Then I went on: "Do you go to the movies?"
"Yes," says he.
"When?", says I.
"In 1920 --- perhaps also in 1930," says he.
"Do you like to read the Sunday comics?"
"Yes," says he, warming up a bit more than usual.
"This is the most important thing yet, doctor," says I. "It shows that me and you are more alike than I thought. And now I want to ask you something more: They tell me that you and Einstein are the only two real sure-enough high-brows and the only ones who can really understand each other. I won't ask you if this is straight stuff for I know you are too modest to admit it. But I want to know this --- Do you ever run across a fellow that even you can't understand?"
"Yes," says he.
"This well make a great reading for the boys down at the office," says I. "Do you mind releasing to me who he is?"
"Weyl," says he.
The interview came to a sudden end just then, for the doctor pulled out his watch and I dodged and jumped for the door. But he let loose a smile as we parted and I knew that all the time he had been talking to me he was solving some problem that no one else could touch.
But if that fellow Professor Weyl ever lectures in this town again I sure am going to take a try at understanding him! A fellow ought to test his intelligence once in a while.
Oh, boy! I've had my fair share of interviews with people who needed some coaxing to spill the beans, but this one takes the cake! Dirac's ability to keep it tight-lipped was on a whole new level. It's like trying to squeeze water from a rock! But hey, that's just how he rolled—mysterious and enigmatic. I guess some minds are just wired differently, and Dirac's was definitely one of a kind!
I know I’m making of Dirac but that’s what he gets for being one of the founders of quantum mechanics. But to make fun of Dirac without mentioning his discoveries would not be fair so here’s a list:
- Dirac equation: A relativistic equation for the electron that correctly predicted the existence of antimatter.
- Quantum electrodynamics: A theory that describes the interaction of light and matter.
- The discovery of the positron: The antiparticle of the electron.
- The development of quantum field theory: A theory that describes the behavior of elementary particles.
- The development of the theory of quantum mechanics: A theory that describes the behavior of matter and energy at the atomic and subatomic level.
And, you know what's intriguing about Dirac? He wasn't just getting a kick out of making people uncomfortable (though he might have found some amusement in it!). No, he had a deep-seated belief in being succinct and speaking only what was absolutely necessary. His mind was wired like that of a true scientist—one who thinks in precise terms and uses language to its fullest potential. Interestingly language, unlike math, which can sometimes be vague and imprecise, Dirac's thoughts were razor-sharp and crystal clear. He had a knack for getting to the heart of the matter without wasting a single word. That was the mark of Dirac’s genius.
Zooming out on Understanding
We have explored particle physics deeply so here’s a table of all particles so we can wrap around our heads this world.
Elementary particles
- Bosons
- Gauge bosons
- Photon - the quantum of EM field
- Gluon - the quantum of the strong force
- W boson - the quantum of the weak force
- Z boson - the quantum of the weak force
- Higgs boson - the quantum of the Higgs field
- Other bosons
- Graviton - the quantum of gravity
- Hypothetical X boson - a boson that could mediate a fifth force of nature
- Hypothetical axion - a boson that could explain the strong CP problem
- Hypothetical sterile neutrino - a neutrino that does not interact with the weak force
- Gauge bosons
- Fermions
- Quarks
- Up quark
- Down quark
- Strange quark
- Charm quark
- Top quark
- Bottom quark
- Leptons
- Electron
- Muon
- Tau lepton
- Electron neutrino
- Muon neutrino
- Tau neutrino
- Other fermions
- Hypothetical Majorana neutrino - a neutrino that could be its own antiparticle
- Hypothetical WIMP - a weakly interacting massive particle that could make up dark matter
- Hypothetical axion - a boson that could explain the strong CP problem
- Quarks
- Composite particles
- Atoms
- Hydrogen atom
- Helium atom
- Lithium atom
- Beryllium atom
- ...
- Molecules
- Water molecule
- Carbon dioxide molecule
- DNA molecule
- Protein molecule
- ...
- Nuclear particles
- Proton
- Neutron
- Deuteron
- Tritium
- Helium-3
- Helium-4
- Composite fermions
- Composite fermion - a hypothetical fermion that could be made up of quarks and gluons
- Hypothetical technicolor fermion - a hypothetical fermion that could be made up of techniquarks
- Hypothetical preon - a hypothetical elementary particle that could make up quarks and leptons
- Atoms
- Other particles
- Hypothetical dark matter particle - a particle that could make up dark matter
- Hypothetical dark energy particle - a particle that could explain dark energy
- Hypothetical string - a hypothetical fundamental unit of matter that could make up all of the other particles
And since this is a book about Lithium, here’s a chart about Lithium:
Lithium
- Isotopes
- Lithium-6 - 3 protons, 3 neutrons
- Lithium-7 - 3 protons, 4 neutrons
- Electrons
- Lithium-6
- 3 electrons
- Lithium-7
- 3 electrons
- Lithium-6
- Forces
- Strong force
- Gluons mediate the strong force between the quarks in lithium
- Weak force
- W and Z bosons mediate the weak force between the quarks in lithium
- Electromagnetic force
- Photons mediate the electromagnetic force between the electrons and protons in lithium
- Strong force
- Gravity
- Gravitons are thought to mediate the force of gravity between all particles
- Universe
- Lithium is thought to have been created in the Big Bang, and it is thought to be distributed throughout the universe
Wow, that was quite a journey into theoretical physics, and to be completely honest, I'm not entirely sure how we ended up there. But that's the beauty of exploration, right?
We followed our intuition and went into whatever caught our interest, and it led us to fascinating places. Now, you might wonder how all this newfound knowledge will help us understand or refine our understanding of lithium better. Well, to be honest, I'm not certain about the exact connection either, but I'm confident it will prove valuable in some way or another. Let's not worry too much about connecting the dots looking forward; instead, let's look back and appreciate the wealth of knowledge we've gained.
It's essential to consider the scale of things. We started with the big picture of lithium, but our curious minds took us all the way down to the super tiny world of particle physics. Now, let's zoom out and take a step back to gain a broader perspective. With this holistic view, we can truly grasp the intricate connections between the tiniest particles and the grandest structures in the universe. It's like viewing the cosmos through a wide-angle lens, allowing us to appreciate the beauty and complexity of the universe at every scale.
Scale is a powerful tool that enhances our comprehension of the world around us. The renowned physicist Richard Feynman was once asked about the meaning of "understanding," and his response couldn't have been more on point:
We can imagine that this complicated array of moving things which constitutes "the world" is something like a great chess game being played by the gods, and we are observers of the game. We do not know what the rules of the game are; all we are allowed to do is to watch the playing. Of course, if we watch long enough, we may eventually catch on to a few of the rules.
The rules of the game are what we mean by fundamental physics. Even if we know every rule, however... what we really can explain in terms of those rules is very limited, because almost all situations are so enormously complicated that we cannot follow the plays of the the game using the rules, much less tell what is going to happen next. We must, therefore, limit ourselves to the more basic question of the rules of the game. If we know the rules, we consider that we "understand" the world.
We all share a common desire to understand ourselves, the world, and the universe. With countless questions and limited answers, physics offers a beautiful pathway to learn and seek understanding.
Perhaps that's why I was naturally drawn to Fermilab, the particle physics accelerator near my house. During my high school years, I spent numerous weekends there, captivated by the mysteries of particle physics. Who knows, I’ll end up a physicist.
I certainly satisfy many of the requirements of the American Physicist in the 20th Century.
The sixty-four-man study which included twenty-two physicists among its "most eminent scientists in the U.S." produced this composite portrait of the American scientist in his prime:
- He is likely to have been a sickly child or to have lost a parent at an early age.
- He has a very high I.Q. and in boyhood began to do a great deal of reading.
- He tended to feel lonely and "different" and to be shy and aloof from his classmates. He had only a moderate interest in girls and did not begin dating them until college.
- He married late ... has two children and finds security in family life; his marriage is more stable than the average. Not until his junior or senior year in college did he decide on his vocation as a scientist.
- What decided him (almost invariably) was a college project in which he had occasion to do some independent research—to find out things for himself. Once he discovered the pleasures of this kind of work, he never turned back.
- He is completely satisfied with his chosen vocation... He works hard and devotedly in his laboratory, often seven days a week.
- He says his work is his life, and he has few recreations... The movies bore him.
- He avoids social affairs and political activity, and religion plays no part in his life or thinking. Better than any other interest or activity, scientific research seems to meet the inner need of his nature.
Clearly, this is close to Robert Oppenheimer. The group studied, like the American physics community then, was predominantly Protestant
From The Making of The Atomic Bomb by Richard Rhodes
The future will tell…
Properties of Lithium
Physical Properties
While I can't physically present Lithium for you to interact with, I'll do my best to immerse you in an experience that captures its essence – from touch to smell, sight, and even the possibility of falling in love with it.
From its soft and sliceable nature to its ability to float on water, lithium is truly a unique element. We'll take a closer look at its shiny appearance, its density that rivals the lightness of wood, and its thermal expansion powers that surpass even those of aluminum and iron.
This is a ride through the wonderful characteristics of lithium!
This is the source from the quotations below.
Lithium metal is soft enough to be cut with a knife. It is silvery-white. In air it oxidizes to lithium oxide. Its melting point of 180.50 °C (453.65 K; 356.90 °F) and its boiling point of 1,342 °C (1,615 K; 2,448 °F) are each the highest of all the alkali metals while its density of 0.534 g/cm3 is the lowest.
Lithium is like the soft butter of metals. You can slice through it with a knife, just like you slice through your favorite creamy spread!
Lithium is as shiny as a disco ball! It gleams like your favorite silver jewelry. When lithium meets the air, it likes to party and turns into lithium oxide. It's like when your food gets all crusty and delicious when you cook it.
Lithium has a very low density (0.534 g/cm3), comparable with pine wood. It is the least dense of all elements that are solids at room temperature; the next lightest solid element (potassium, at 0.862 g/cm3) is more than 60% denser. Apart from helium and hydrogen, as a solid it is less dense than any other element as a liquid, being only two-thirds as dense as liquid nitrogen (0.808 g/cm3). Lithium can float on the lightest hydrocarbon oils and is one of only three metals that can float on water, the other two being sodium and potassium.
Lithium is lighter than a feather! It's even less dense than a solid piece of pine wood. It's like carrying around a cloud in your hand. Lithium loves to swim! It can float on water just like a rubber duck. It's a special metal that knows how to stay afloat!
Lithium's coefficient of thermal expansion is twice that of aluminium and almost four times that of iron. Lithium is superconductive below 400 μK at standard pressure and at higher temperatures (more than 9 K) at very high pressures (>20 GPa). At temperatures below 70 K, lithium, like sodium, undergoes diffusionless phase change transformations. At 4.2 K it has a rhombohedral crystal system (with a nine-layer repeat spacing); at higher temperatures it transforms to face-centered cubic and then body-centered cubic. At liquid-helium temperatures (4 K) the rhombohedral structure is prevalent. Multiple allotropic forms have been identified for lithium at high pressures.
When it comes to expanding with heat, lithium isn't afraid to take extra space! It expands even more than those stretchy yoga pants. Lithium becomes a supercool superhero at really, really cold temperatures. It conducts electricity with superpowers, just like your favorite comic book characters!
Lithium has a mass specific heat capacity of 3.58 kilojoules per kilogram-kelvin, the highest of all solids. Because of this, lithium metal is often used in coolants for heat transfer applications.
Lithium is the champion of heat capacity! It can hold onto heat like nobody's business. It's like wearing the warmest, coziest sweater on a chilly day!
Chemistry of Lithium
If we talk about its physical properties, we cannot leave out its chemistry!!!
This is the source from the quotations below.
Lithium reacts with water easily, but with noticeably less vigor than other alkali metals. The reaction forms hydrogen gas and lithium hydroxide. When placed over a flame, lithium compounds give off a striking crimson color, but when the metal burns strongly, the flame becomes a brilliant silver. Lithium will ignite and burn in oxygen when exposed to water or water vapor. In moist air, lithium rapidly tarnishes to form a black coating of lithium hydroxide (LiOH and LiOH·H2O), lithium nitride (Li3N) and lithium carbonate (Li2CO3, the result of a secondary reaction between LiOH and CO2). Lithium is one of the few metals that react with nitrogen gas.
Lithium is a super quirky element and loves to react with water, but it's not as wild as its alkali metal buddies. When lithium meets water, it creates hydrogen gas and lithium hydroxide.
But watch out, if you set it on fire, the flame turns into a dazzling silver show! Lithium also has a unique relationship with nitrogen, and it's one of the few metals that can't resist its charm. It's like the cool kid at the periodic table party, always up for an exciting chemical adventure!
Because of its reactivity with water, and especially nitrogen, lithium metal is usually stored in a hydrocarbon sealant, often petroleum jelly. Although the heavier alkali metals can be stored under mineral oil, lithium is not dense enough to fully submerge itself in these liquids.
Lithium has some peculiar habits when it comes to storage. It prefers to hang out in a hydrocarbon sealant, like petroleum jelly, to keep its reactivity in check. Unlike its heavier alkali metal pals, lithium isn't dense enough to fully immerse itself in mineral oil. It's like a swimmer who needs a little more buoyancy to stay afloat.
Lithium has a diagonal relationship with magnesium, an element of similar atomic and ionic radius. Chemical resemblances between the two metals include the formation of a nitride by reaction with N2, the formation of an oxide (Li2O) and peroxide (Li2O2) when burnt in O2, salts with similar solubilities, and thermal instability of the carbonates and nitrides. The metal reacts with hydrogen gas at high temperatures to produce lithium hydride (LiH).
If you thought lithium's chemistry couldn't get any more interesting, think again! It has a special bond with magnesium, their atomic and ionic radii match perfectly. They have a lot in common, from forming nitrides when they meet nitrogen to creating oxides and peroxides when they dance with oxygen. They even share similar solubilities and a knack for thermal instability. It's like they're chemistry's power couple, always making waves together!
Lithium forms a variety of binary and ternary materials by direct reaction with the main group elements. These Zintl phases, although highly covalent, can be viewed as salts of polyatomic anions such as Si44-, P73-, and Te52-. With graphite, lithium forms a variety of intercalation compounds.
Lithium isn't shy when it comes to making new compounds. It loves to mingle with main group elements, forming a variety of binary and ternary materials. These compounds, called Zintl phases, are like the fancy salts of the chemical world. They have intriguing polyatomic anions that make them stand out from the crowd. Lithium also knows how to groove with graphite, forming cool intercalation compounds. It's like being part of an exclusive dance club where lithium gets its groove on!
Inorganic compounds Lithium forms salt-like derivatives with all halides and pseudohalides. Some examples include the halides LiF, LiCl, LiBr, LiI, as well as the pseudohalides and related anions. Lithium carbonate has been described as the most important compound of lithium. This white solid is the principal product of beneficiation of lithium ores. It is a precursor to other salts including ceramics and materials for lithium batteries. The compounds LiBH4 and LiAlH4 are useful reagents. These salts and many other lithium salts exhibit distinctively high solubility in ethers, in contrast with salts of heavier alkali metals. In aqueous solution, the coordination complex [Li(H2O)4]+ predominates for many lithium salts. Related complexes are known with amines and ethers.
Lithium is quite the matchmaker when it comes to forming compounds with halides and pseudohalides. It can cozy up with halogens like fluorine, chlorine, bromine, and iodine, creating salt-like derivatives such as LiF, LiCl, LiBr, and LiI.
But it doesn't stop there! Lithium also gets along with pseudohalides and their related anions, expanding its chemical dating pool. It's like being the ultimate chemistry matchmaker, bringing together different elements to create new and exciting compounds.
Among all its compounds, lithium carbonate takes the crown as the most important one. This white solid is a superstar in the world of lithium. It's the star product of the beneficiation of lithium ores, making it a valuable precursor for other salts, ceramics, and materials used in lithium batteries. It's like the celebrity of lithium compounds, stealing the spotlight with its versatile applications.
But wait, there's more! Lithium has a couple of friends who are incredibly useful reagents: LiBH4 and LiAlH4. These salts are like trusty sidekicks, helping chemists in various reactions. What makes them even more special is their distinctively high solubility in ethers, unlike the salts of heavier alkali metals. It's like having a secret weapon that dissolves effortlessly in a magical solvent, ready to tackle any chemical challenge.
When lithium salts take a dip in aqueous solution, they often form a coordination complex called [Li(H2O)4]+. It's like throwing a pool party where lithium is surrounded by water molecules, having a grand old time. But lithium is a social butterfly and can also form related complexes with amines and ethers. It's like being the life of the party, mingling with different guest molecules to create a chemical atmosphere.
The inorganic compounds of lithium are like a chemistry party, where lithium plays matchmaker, showcases its superstar compound, hangs out with useful reagents, and forms fascinating complexes with various guest molecules. It's a world full of salty connections and electrifying interactions, making lithium's chemistry pretty cool.
Organic chemistry Organolithium compounds are numerous and useful. They are defined by the presence of a bond between carbon and lithium. They serve as metal-stabilized carbanions, although their solution and solid-state structures are more complex than this simplistic view. Thus, these are extremely powerful bases and nucleophiles. They have also been applied in asymmetric synthesis in the pharmaceutical industry. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. Like its inorganic compounds, almost all organic compounds of lithium formally follow the duet rule (e.g., BuLi, MeLi). However, it is important to note that in the absence of coordinating solvents or ligands, organolithium compounds form dimeric, tetrameric, and hexameric clusters (e.g., BuLi is actually [BuLi]6 and MeLi is actually [MeLi]4) which feature multi-center bonding and increase the coordination number around lithium. These clusters are broken down into smaller or monomeric units in the presence of solvents like dimethoxyethane (DME) or ligands like tetramethylethylenediamine (TMEDA). As an exception to the duet rule, a two-coordinate lithate complex with four electrons around lithium, [Li(thf)4]+[((Me3Si)3C)2Li]–, has been characterized crystallographically.
Lithium's chemistry extends to organic chemistry as well.
What’s organic chemistry?
The study of carbon-containing compounds, which are the building blocks of life and many things around us. Carbon is the superstar of organic chemistry because it can form a wide variety of molecules by bonding with other atoms. Organic chemistry is essential in our world because it helps us understand how living organisms function, how drugs interact wit our bodies, how materials are made, and even how food is cooked.
The word "organic" in organic chemistry might make you think of organic food, right? But here's the interesting part: The word "organic" in organic chemistry actually comes from a historical perspective. Back in the day, people thought that compounds derived from living organisms had a special "vital force" that made them different from compounds obtained from non-living sources. They believed this vital force was responsible for the complex chemistry observed in living organisms. As a result, they called these compounds "organic" to highlight their connection to life. Next time you hear organic chemistry, don’t freak out and just think about the fascinating study of carbon compounds.
What about about inorganic chemistry? It’s the branch of chemistry that deals with elements and compounds that don’t contain carbon. While organic chemistry focuses on carbon-containing compounds, inorganic chemistry explores everything else in the vast field of chemistry. Inorganic chemistry is all about metals, minerals, ceramics, catalysts, semiconductors, and more as it explores the world of compounds beyond carbon.
Anyways, organic chemistry can help us understand lithium’s chemistry.
Lithium forms interesting bonds with carbon, giving birth to organolithium compounds. These compounds are like supercharged versions of themselves, with amazing base and nucleophilic powers. They're the superheroes of organic synthesis, saving the day in pharmaceutical labs. But watch out, they can be a bit fiery and need to be handled with care. It's like having a powerful ally who can turn the tide in any chemical battle!
One eccentric thing about lithium's organic compounds is that they like to break the rules. Most of them follow the "duet rule," but in the absence of coordinating solvents or ligands, they form larger clusters with fancy multi-center bonding. It's like they're throwing a spontaneous party and inviting all their friends to join in the fun! But when solvents or ligands come into play, the clusters break down into smaller units, like a party that gets more intimate and cozy. It's like finding the perfect balance between a wild bash and a chill gathering.
History of Lithium
How Language Affects Understanding
Before we get into the history of lithium, I'd like to take a moment to appreciate the beauty and complexity of language and its impact on our understanding. Through my study of lithium, I've come to realize how easily we get fixated on specific associations and abstractions, often overlooking the raw essence of these elements.
For many of us, lithium is synonymous with batteries and its symbol "Li" in the periodic table. We might know its atomic number is 3, but the true experience of early discoverers was quite different. To them, lithium wasn't just an abstract concept; it was a silvery substance soft enough to be cut with a knife. They might have even playfully referred to it as "The Silver Cheese."
Why does this matter? Understanding the origin of words like "lithium" allows us to see beyond the common associations and preconceived notions. It helps us strip away the dogma and truly see these elements for what they are, not just the concepts they've become in our minds. By exploring the roots of these names and imagining what the early discoverers had in mind, we can appreciate the true essence of each element.
If you're not a miner, lithium refiner, scientist, or someone living near lithium deposits, your perception of lithium might be limited to its association with batteries, missing out on its essense as "The Silver Cheese!"
The Discovery of Lithium
In the early exploration of lithium, the discovery and understanding of this unique element unfolded gradually, emerging from prehistoric knowledge and limited awareness. A crucial mineral in this journey was petalite (LiAlSi4O10), first unearthed in 1800 by Brazilian chemist and statesman José Bonifácio de Andrada e Silva on the picturesque island of Utö in Sweden. This find laid the foundation for subsequent investigations that led to the identification and understanding of lithium.
In 1817, Swedish chemist Johan August Arfwedson conducted a meticulous examination of petalite. During this analysis, he exposed a sample to a flame, expecting to see the familiar orange color characteristic of sodium. However, the flame displayed a vibrant crimson hue, capturing his attention. In that serendipitous moment, Arfwedson realized he had stumbled upon something extraordinary—an entirely new alkali metal. This momentous discovery marked just the beginning of our journey into the world of lithium.
Arfwedson's groundbreaking discovery opened the door to further exploration and research into lithium. Scientists soon realized that lithium formed compounds similar to those of sodium and potassium, but with distinct properties. For instance, its carbonate and hydroxide displayed lower solubility in water and were less alkaline compared to the corresponding compounds of sodium and potassium.
In admiration of this new alkali material, the chemist Jöns Jakob Berzelius gave it the names "lithion" or "lithina," both derived from the Greek word "lithos," meaning "stone." These names symbolized its discovery within a solid mineral, distinguishing it from potassium derived from plant ashes and sodium abundant in animal blood. This recognition emphasized the unique nature of lithium as an element found in solid form, adding to its allure and scientific significance.
In 1821, William Thomas Brande, drawing inspiration from the electrolysis methods used by the renowned chemist Sir Humphry Davy to isolate potassium and sodium, successfully applied the same technique to isolate lithium oxide and obtain the pure metal. Brande's accomplishments did not stop there; he also described various pure salts of lithium, including the chloride. Additionally, he estimated the atomic weight of lithium to be approximately 9.8 g/mol based on his calculations of the metal content in lithia.
However, as scientific understanding advanced and more precise measurements were made, the modern value of lithium's atomic weight was later refined to approximately 6.94 g/mol. This progression in scientific knowledge underscores the continuous refinement and improvement that accompanies scientific exploration and discovery.
In 1855, further advancements in the understanding of lithium occurred when Robert Bunsen and Augustus Matthiessen successfully carried out the electrolysis of lithium chloride on a larger scale. This breakthrough enabled the production of significant quantities of lithium. Building upon their discovery, the German company Metallgesellschaft AG achieved a pivotal milestone in 1923 by commencing commercial production of lithium. They utilized electrolysis on a liquid mixture of lithium chloride and potassium chloride, paving the way for widespread use and application of this remarkable element.
The early history of lithium showcases human curiosity and ingenuity, with scientists like Arfwedson, Brande, Bunsen, and Matthiessen unraveling the element's mysteries. From the surprise of a vibrant crimson flame to the triumph of isolating the pure metal, each step in the journey brought us closer to understanding the properties and potential applications of lithium. These early discoveries laid the groundwork for subsequent developments and the exploration of lithium's vast potential in various fields. The story of lithium's discovery serves as a reminder of science's remarkable journey, continually unearthing the hidden treasures of the natural world.
Lithium's Applications: Batteries, Drugs, and Atomic Bombs
Lithium has left its mark on multiple fields, ranging from medicine to batteries. It's a true multitasker! Let's go on a journey through the applications of this extraordinary element.
Lithium has had quite the resume, from being used to treat mania to being used as greases for aircraft engines during World War II. But that's not all—did you know lithium has also found its way into the world of nuclear physics?
During the Cold War, the demand for lithium skyrocketed due to the production of nuclear fusion weapons. An isotopes of lithium, lithium-6, played a crucial role in this context, as it could produce tritium when exposed to neutrons. Tritium is a key component of hydrogen bombs, making these lithium isotopes invaluable for their production. The United States emerged as a leading lithium producer during this period, amassing an astounding 42,000 tonnes of lithium hydroxide.
Now, the first major application of Lithium was not in nuclear physics but rather in high-temperature lithium greases, keeping aircraft engines running smoothly during World War II. Lithium-based soaps proved to be superior, thanks to their higher melting point and lower corrosiveness compared to other options. These greases helped keep the skies friendly and the machines humming.
However, lithium didn't stop there. It made its mark in the realm of pharmaceuticals and medicine. Lithium carbonate, with its mood-stabilizing properties, became a game-changer in the treatment of bipolar disorder. Thanks to the pioneering work of Australian psychiatrist John Cade, lithium regained its place in the medical world, taming the wild swings of mania and providing much-needed stability to patients. Additionally, lithium orotate, a different form of lithium, has shown potential cognitive and neuroprotective benefits, although more research is needed to fully understand its effects.
The multifaceted nature of lithium continues to intrigue scientists and researchers, leading to new discoveries and applications across a variety of fields. From powering aircraft engines to stabilizing moods, lithium has proven to be an indispensable element with profound contributions to humanity. As our understanding of lithium grows, so does our appreciation for its remarkable capabilities and potential future applications.
You may ask, WHAT ABOUT BATTERIES? Yes, batteries are important but they’re merely one of Lithium’s applications.
The development of lithium-ion batteries revolutionized the demand for lithium and became the dominant use in the 2000s. With the surge of lithium demand for batteries, new companies expanded brine isolation efforts to meet the rising demand. The versatility and energy storage capabilities of lithium-ion batteries fueled the growth of portable electronics, electric vehicles, and grid-scale energy storage systems. This advancement in battery technology has significantly diversified the applications and importance of lithium in our modern society.
But that’s not all! We're about to explore the diverse applications of lithium in batteries, ceramics, aerospace, metallurgy, and much more. Lithium truly knows how to wear many hats and make its mark in the world of science and technology.
I. Batteries and Energy
In the section after Applications, we will look at lithium-ion cells, batteries, and more detail.-
Portable electronics (e.g., smartphones, laptops): Lithium-ion batteries power our everyday devices by storing and releasing electrical energy efficiently. Think of them as the fuel tanks for your smartphones and laptops, allowing you to use them on the go without constantly plugging them in.
-
Electric vehicles (EVs) and hybrid vehicles: Lithium-ion batteries play a crucial role in the electrification of transportation. They provide the energy needed to propel electric and hybrid vehicles, allowing them to travel longer distances and reduce dependence on fossil fuels.
-
Energy storage systems (grid-scale and residential): These systems use lithium-ion batteries to store excess energy generated by renewable sources like solar and wind. It's like having a giant rechargeable battery that can store electricity and release it when needed, helping to balance the supply and demand of energy.
II. Pharmaceuticals and Medicine
A. Lithium Carbonate
-
Treatment for bipolar disorder: Lithium carbonate has been widely used as a treatment for bipolar disorder since its reintroduction in the late 1940s. Australian psychiatrist John Cade is credited with reintroducing and popularizing the use of lithium for treating mania. The mood stabilizing properties of lithium help to regulate mood swings and reduce the intensity and frequency of manic and depressive episodes in individuals with bipolar disorder.
-
Mood stabilizer: Lithium carbonate acts as a mood stabilizer by modulating the levels of certain neurotransmitters in the brain, such as serotonin and norepinephrine. It helps to balance the excitatory and inhibitory signals in the brain, promoting a more stable mood state.
B. Lithium Orotate
- Potential cognitive and neuroprotective benefits: Lithium orotate is an alternative form of lithium that is suggested to have potential cognitive and neuroprotective benefits. Some studies have explored its potential in improving cognitive function and protecting the brain from neurodegenerative disorders. However, it's important to note that the scientific evidence supporting these claims is limited, and more research is needed to establish its effectiveness.
C. Research into other medical applications
Ongoing research explores the potential of lithium in other medical applications, including its effects on neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease.
Scientists are studying the mechanisms through which lithium may exert neuroprotective effects and its potential to modulate signaling pathways involved in disease progression.
III. Ceramics and Glass
A. Lithium-based Ceramics
-
Heat-resistant materials: Lithium-based ceramics are known for their excellent heat resistance properties. They can withstand high temperatures without deforming or breaking, making them suitable for applications in high-temperature environments like furnaces and aerospace components.
-
Batteries and fuel cells (ceramic electrolytes): Lithium-based ceramics are used as solid electrolytes in certain types of batteries and fuel cells. These ceramics enable efficient ion transport, enhancing the performance and safety of energy storage and conversion devices.
B. Glass and glass-ceramics:
- High-performance glass (e.g., display screens, lenses): Lithium compounds are used in the production of high-performance glass, such as display screens for electronic devices and lenses for optical systems. These glasses offer excellent clarity, durability, and scratch resistance, providing us with clear and vibrant visual experiences.
- Cookware and glassware (e.g., thermal shock resistance): Lithium-based glass-ceramics are highly resistant to thermal shock, meaning they can withstand rapid temperature changes without breaking. This property makes them ideal for cookware and glassware, as they can go from hot to cold (and vice versa) without shattering.
IV. Lubricants and Greases
A. Lithium-based Lubricants
-
High-temperature applications: Lithium-based lubricants are used in high-temperature environments where other lubricants may fail. They provide excellent lubrication and reduce friction, preventing wear and extending the lifespan of moving parts in machines and engines.
-
Automotive and industrial lubrication: Lithium-based lubricants find widespread use in automotive and industrial applications, such as engine oils, gear lubricants, and greases for bearings and joints. They ensure smooth operation and protect against wear and corrosion.
B. Greases (thickened with lithium hydroxide or lithium soap)
Lithium hydroxide or lithium soap can be used to thicken lubricants into greases. Greases have a semi-solid consistency, allowing them to stay in place and provide long-lasting lubrication in applications where oils may not be suitable.
V. Metallurgy and Alloys
A. Lithium-aluminum alloys
- Lightweight structural materials: Lithium-aluminum alloys combine the lightweight properties of lithium with the strength of aluminum, resulting in lightweight structural materials. These alloys find applications in aerospace and automotive industries, where weight reduction is critical for fuel efficiency and performance. Lithium-aluminum alloys are used in the manufacturing of aircraft components, such as wings and fuselage structures, as well as in automotive parts like body panels, frames, and engine components.
B. Lithium-based alloys
-
High-performance materials: Lithium can form alloys with various metals, resulting in high-performance materials with unique properties. These alloys can exhibit enhanced strength, conductivity, and corrosion resistance, making them valuable in industries such as electronics, aerospace, and manufacturing.
-
Research into improved properties: Ongoing research aims to further optimize the properties of lithium-based alloys and explore their potential in areas like additive manufacturing (3D printing) and advanced materials development.
C. Welding
Lithium is sometimes used as a welding flux, which helps facilitate the welding process by removing impurities and preventing oxidation of the metal during welding operations.
VI. Catalysts and Chemical Synthesis
A. Lithium compounds as catalysts in chemical reactions
Certain lithium compounds can act as catalysts, facilitating chemical reactions by increasing reaction rates or enabling specific transformations. They play an important role in organic synthesis and industrial processes, improving efficiency and selectivity.
B. Chemical synthesis applications
Lithium compounds find applications in various chemical synthesis processes, such as the production of pharmaceuticals, polymers, and specialty chemicals. They can act as reagents, initiators, or intermediates, contributing to the synthesis of desired molecules and materials.
VII. Other Applications
A. Air purification systems
Lithium chloride-based systems are used for air purification, particularly in environments where moisture and humidity control are critical. Lithium chloride can absorb excess moisture from the air, helping to maintain desired humidity levels and purify the air.
B. Desiccants and drying agents
Lithium compounds, such as lithium bromide, have desiccant properties, meaning they can absorb moisture from the surrounding environment. They are used in various applications, including drying agents for air conditioning systems and moisture control in manufacturing processes.
C. Specialty polymers and resins
Lithium compounds are utilized in the production of specialty polymers and resins with specific properties, such as enhanced flame retardancy, electrical conductivity, or thermal stability. These polymers find applications in electronics, automotive, and construction industries.
D. Nuclear power generation (lithium-6 for tritium production)
Lithium-6, the less common isotope of lithium, plays a crucial role in nuclear power generation by producing tritium, a key component in the fusion reaction of hydrogen isotopes. Tritium finds applications in the production of thermonuclear weapons and holds promise for future applications in fusion power plants.
We will explore Lithium-6 and its uses in nuclear physics in more detail in the following section.
VIII. Future Applications
Scientists, engineers, and technologists continue to explore and develop new applications and technologies involving lithium. This includes advancements in battery technology, medical research, materials science, and other fields. Ongoing research is exciting and who knows what the future holds for lithium! Let's use our imagination and creativity to envision the future of our world with lithium.
Nuclear Physics: Lithium-6, Fusion, Reactors, and The Future
I want to begin on nuclear fusion and how it all works.
Fusion reactors are an extraordinary power source that exploit the energy released when tiny atomic particles, such as deuterium and tritium, join together. It's a tough challenge, but once they get the fusion fire burning, it's ignition time!
Now, there's a bit of a puzzle. Deuterium, found in water, is pretty common on Earth, so we're good on that front. But tritium, well, that's a different story. It's produced artificially, usually as a byproduct of certain nuclear reactors. And guess what? We might face a tritium shortage when some of those reactors retire in the next decade or so.
In the fusion world, there are four fusion reactions, but the real star is the D-T reaction. When deuterium and tritium hold hands and fuse, they produce helium and release a mind-blowing amount of energy. We're talking about an energy yield per atomic mass that's way higher than what you get from regular fission reactions.
But hold your horses; there's a twist. Fusion reactions are a bit picky. Other reactions, like D-D, are shy and hardly ever happen. So we're all about the D-T reactions because it has the highest probability of giving us the energy we crave.
The journey to achieve fusion ignition has been a rollercoaster ride. We've seen some pretty impressive moments in labs, like the Tokamak Fusion Test Reactor and the Joint European Torus, where they danced with D-T reactions and released fusion energy like pros. But we're still working on getting more energy out than we put in. It's a tricky game, but we're making progress.
Now, here's the cool part: fusion reactors have this safety feature. If things go haywire, they'll just shut down on their own, no big explosions. But there's a tiny catch. Fusion does produce some low-level radioactive waste, so it's not entirely clean.
The future of fusion is like a thrilling suspense novel. We've made progress in the lab, but a utility-scale fusion power plant is still on our to-do list. We know it'll take some time, but we're not giving up. In the meantime, fission reactors are still holding the fort, and they might team up with fusion in fancy fusion-fission hybrid reactors.
So, that’s where fusion stands, aiming to be the top energy source. How far away? No one really knows but one day, fusion power will give us energy like never before and the world will never look back.
I told about about the D-T Reaction but there are a few more so let me take you around the different fusion reactions and stop by to ask a few questions.
-
D-D Reaction (Deuterium-Deuterium Reaction): In this fusion reaction, two deuterium nuclei (deuterons) join together to create helium-3 and release a high-energy neutron. It's like a cute fusion tango between two deuterium atoms. However, D-D reactions have a low probability of happening, so they don't really steal the show in most fusion scenarios.
-
D-T Reaction (Deuterium-Tritium Reaction): Now, this is where the real party starts! The D-T reaction is the star of the fusion show. A deuterium nucleus and a tritium nucleus come together in a fiery embrace, and they produce helium-4 (the happy helium we know) and a super-fast neutron. This fusion reaction is the one we're most interested in because it has a much higher probability of happening compared to D-D reactions.
-
D-He3 Reaction (Deuterium-Helium-3 Reaction): This fusion trio involves a deuterium nucleus and a helium-3 nucleus merging together, creating a helium-4 nucleus and a snazzy proton. While it's a cool dance, it's not as common as the D-T reaction. So, it's like a special fusion performance that doesn't steal the spotlight.
-
T-T Reaction (Tritium-Tritium Reaction): Last but not least, we have the T-T reaction, where two tritium nuclei come together in a fusion fiesta. They produce helium-4 and two fast neutrons. While this reaction does happen, it's even less frequent than the D-D reactions. So, it's like a rare fusion duet that doesn't show up on stage too often.
The D-T reaction is the most popular in the world of fusion, stealing the hearts of researchers with its high energy yield and potential for creating a clean and abundant power source. But hey, scientists are always up for a challenge, and they're working on mastering all the fusion moves to create a sustainable energy future for all of us! For instance, one of the leading Fusion startups are going with the D-He3 reaction while the D-T reactions remains to be the most promising.
If we talk about nuclear reaction, we also need to talk about reactors and their future.
Nuclear Reactors
Nuclear reactors are the center of a nuclear power plant. How it works is simple. First, they control the nuclear chain reactions that produce heat through fission. After the heat is produced, the heat is used to create electricity by spinning turbines.
Let’s jump in.
We have these Generation 3 (Gen 3) reactors, and they're cool because they're passively safe. You don't need someone to be pressing buttons or keeping the water running all the time. Nature does its thing, using convection to handle waste heat if anything goes wrong. Safety is on point with Gen 3, but they still have the same old efficiencies and designs, mostly relying on uranium-235 for fission.
But here's where it gets interesting – Generation 4 (Gen 4) reactors change everything. The biggest thing is the efficiency. Current reactors run at lower temperatures, which means they're not super efficient. But Gen 4 reactors want to crank up the heat and boost that efficiency, baby!
You have all sorts of cool concepts being explored for Gen 4. Some reactors are like, "Hey, forget water! Let's use gas as our coolant!" Yeah, helium-cooled and gas-cooled reactors are in the mix. And listen to this – they're even working on reactors that can whip up hydrogen straight up without going through the whole steam-making thing. How cool is that?
Oh, and there’s also this one! Instead of water, you can use liquid metal coolants like lead, sodium, or even salt. Totally changing how traditional reactors!
Now, hold on, there's more! There's this super cool concept called the pebble bed reactor. Picture this – you have these tiny baseball-sized pebbles, and inside them, there's uranium fuel and moderator mixed in. It's like a nuclear party in there! These pebbles are totally safe because they won't go crazy with the nuclear stuff if they fall down. They're chill. And the best part is, they're continuously refueled, so no more long shutdowns for refueling! That's what I'm talking about!
The future's still in progress in research labs and test facilities, but these Gen 4 reactors are definitely raising eyebrows and sparking excitement. Imagine a world with more efficient, safer, and greener nuclear power!
That’s not all, there’s another reactor that seems promising and it’s gaining traction every year with new methods so it’s a matter of time until we figure it out.
Introducing the Hybrid Fusion-Fission Reactor!
Hybrid Fusion-Fission Reactor
In a typical nuclear fission reactor, uranium or plutonium atoms undergo a process called fission, resulting in the release of energy in the form of heat. This heat is utilized to produce steam, which drives turbines and generates electricity. However, in a hybrid fusion-fission reactor, we bring fusion.
As discussed previously, fusion is the process of combining two light atomic nuclei, typically isotopes of hydrogen, to form a heavier nucleus. This fusion process releases an immense amount of energy, much more than what is released in fission reactions. The fuel used for fusion is often isotopes of hydrogen, such as deuterium and tritium.
Simply put, the hybrid concept uses a special hug between atoms (fusion) to make fast particles (neutrons), which help control a safe way of breaking atoms apart (fission) to create clean and powerful energy.
THe basic idea is to use high-energy fast neutrons from a fusion reactor to trigger fission.
Here's how it works in the hybrid reactor:
-
Fusion: The fusion reaction is initiated by heating and compressing a mixture of deuterium and tritium using powerful energy sources like lasers or magnetic confinement. This creates a high-temperature and high-pressure environment similar to the conditions in the core of stars.
-
Energy Release: The deuterium and tritium isotopes combine, or fuse, to form helium and release an incredible amount of energy in the process. This energy is in the form of fast-moving particles, mainly neutrons, and high-energy photons (gamma rays).
-
Neutron Activation: The high-energy neutrons produced during fusion can interact with other materials in the reactor, such as lithium, which surrounds the fusion region. The lithium can capture these neutrons and undergo a reaction called neutron activation, producing tritium and helium.
-
Fission Reaction: The tritium produced in the neutron activation process is a valuable fuel for a fission reaction. In the hybrid reactor, this tritium can be used to fuel a secondary fission reaction. The tritium is combined with uranium or thorium fuel, and when it undergoes fission, it releases additional energy and produces new neutrons.
-
Energy Generation: The fission process releases heat, similar to that in a conventional nuclear reactor. This heat is then utilized to generate steam and produce electricity through traditional turbine generators.
The beauty of the hybrid fusion-fission reactor lies in its ability to use the energy from the fusion reaction to initiate and sustain the fission reaction, creating a self-sustaining and highly efficient energy generation system.
This combined fusion-fission approach holds the promise of abundant, clean, and reliable energy generation, making significant strides towards a sustainable and greener energy future.
Researchers get excited about hybrid fusion-fission reactors because they can potentially produce more energy than either fusion or fission reactors alone, and if that wasn’t enough, they can also help in reducing the amount of radioactive waste generated by conventional nuclear power plants. By utilizing nuclear waste as part of the blanket material and converting it into useful fuel, the reactor contributes to waste management and minimizes its environmental impact.
What is the role of lithium?
Lithium is the special ingredient in the hybrid fusion-fission reactor, acting as both a facilitator and a fuel source:
-
Neutron Moderator: Lithium slows down fast-moving neutrons produced during fusion, making them more effective in triggering fission reactions. By moderating these neutrons, lithium increases their chances of interacting with uranium or thorium fuel, which leads to more efficient and controlled fission reactions.
-
Tritium Production and Breeding: The fusion reaction generates high-energy neutrons that can interact with lithium. Through a process called neutron activation, lithium captures these neutrons and transforms into tritium, an isotope of hydrogen. Tritium is a valuable fuel for the fission reaction, and its production is crucial for sustaining the fission process. Moreover, some of the neutrons from fission can also interact with lithium to produce additional tritium through tritium breeding, ensuring a continuous supply of fuel.
Lithium plays a crucial role as both a mediator and a fuel source in the hybrid fusion-fission reactor. It serves to moderate the fast neutrons generated during fusion, enhancing their efficiency in inducing fission reactions. Moreover, lithium captures high-energy neutrons from the fusion process, leading to the production of tritium, which in turn acts as fuel for the fission reaction. This dual function of lithium is vital as it enables the capture of energy from both fusion and fission, creating an immensely efficient and sustainable energy generation system.
It's worth noting that hybrid fusion-fission reactors are still in the developmental phase. Although there are several current challenges, I won't deny that the list is extensive and may seem insurmountable, the keyword here is "seem."
One way towards progress is the convergence of technologies. I’ll give you an example.
AI has been used to control nuclear fusion in a tokamak reactor, marking a crucial step in unlocking the potential of clean and abundant fusion energy.
The company Google DeepMind collaborated with the Swiss Plasma Center to develop an AI system for effectively managing the complex and continuously changing plasma inside the tokamak. Using deep reinforcement learning, the AI autonomously manipulates the reactor's 19 magnetic coils to shape the plasma into desired configurations.
This groundbreaking achievement opens the door to designing more efficient and powerful tokamaks, propelling us further on the path to viable fusion reactors. With AI-controlled tokamaks, we can optimize heat transfer, prevent plasma instabilities, and explore new possibilities that push the boundaries of fusion power research.
I can easily see how we will design new algorithms to help us with the hybrid fusion-fission reactor.
If the solution can be discovered, it will be discovered. I have no doubt, with the right approach, we will have abundant and cheap energy in the near-term future.
To get to that future, we might need of lots of lithium-6 so let’s learn all about it from its history to a possible trillion-dollar business opportunity.
The Separation of Lithium-6
I attended a 4th of July party where I had the chance to meet a nuclear physicist working at a leading nuclear fusion company. As we chatted, I excitedly shared my thoughts about The Cheesy Energy of Lithium.
Intrigued by my enthusiasm and ambition, he presented me with a challenge: the separation of lithium-6.
He explained that fusion reactors required lithium-6, the less common isotope, to produce tritium. However, the process of separating it is incredibly difficult, and anyone who can find a solution to increase the supply of lithium-6 would stand to make billions of dollars. "Well, that's certainly an intriguing problem," I thought to myself, ready to dive into the challenge.
Why is this important? Well, it all comes down to the abundance of lithium-6 in nature, which is quite rare compared to lithium-7 (making up 92.5% of natural lithium). So, if we want to use lithium-6, we need to find a reliable source for it.
Now, you might wonder, "Why can't we just use lithium-7 instead?" The reason lithium-6 is specifically needed for certain applications, like fusion reactions, is because it has a higher probability of undergoing nuclear reactions when exposed to neutrons. This is because the nucleus of lithium-6 is more unstable than the nucleus of lithium-7. When a neutron hits a lithium-6 atom, it can cause the nucleus to split, releasing energy and producing tritium, a radioactive isotope of hydrogen. Tritium can then be used to fuel fusion reactions.
The cross-section of the lithium-6 neutron reaction is about 940 barns, while the cross-section of the lithium-7 neutron reaction is only about 14 barns. This means that a lithium-6 atom is about 67 times more likely to undergo a nuclear reaction when exposed to a neutron than a lithium-7 atom.
This difference in cross-sections is why lithium-6 is specifically needed for certain applications, like fusion reactions. In order to make a fusion reactor self-sufficient in tritium, it needs to be able to breed more tritium than it consumes. This is only possible if the fusion reactor is using lithium-6, as the lithium-7 neutron reaction does not produce enough tritium to be self-sufficient.
Nuclear reactions, such as neutron capture or nuclear fission, occur when a nucleus interacts with a neutron. The probability of a nuclear reaction taking place depends on the availability of unoccupied energy levels or resonances within the nucleus. In the case of lithium-6, its lower neutron-to-proton ratio allows for more favorable energy configurations, creating a higher probability of capturing an incoming neutron.
This phenomenon is related to the concept of nuclear binding energy. Nuclei are held together by the strong nuclear force, which overcomes the electrostatic repulsion between protons. The energy required to separate a nucleus into its individual nucleons (protons and neutrons) is known as the binding energy. Certain nuclei have a lower binding energy per nucleon compared to others, making them more susceptible to nuclear reactions.
In the case of lithium-6, its lower neutron-to-proton ratio contributes to a less stable configuration compared to lithium-7. This lower stability leads to a higher binding energy per nucleon for lithium-6, making it more likely to undergo nuclear reactions when exposed to neutrons. The capture of a neutron can result in the formation of lithium-7 or tritium, depending on the specific reaction and conditions.
But also the probability is affected by other factors as well such as the energy of the incoming neutrons, the presence of other isotopes or impurities, and the specific nuclear reaction mechanisms.
Those are the reasons lithium-6 will be important (and a possible headache for future nuclear fusion reactors). We better avoid that future and figure out better ways to separate lithium-6.
What are the different ways to separate lithium-6?
The separation of lithium-6 from natural lithium, which predominantly consists of lithium-7, can be achieved through multiple methods. Let’s start with the most popular.
-
Column exchange (COLEX) separation uses mercury to separate lithium-6. The process involves passing a mixture of isotopes through a column packed with an ion exchange material. The material selectively binds one isotope more strongly than the other, allowing for their separation. The separated isotope can then be recovered by eluting it from the column. When added to solutions containing lithium hydroxide, lithium-6 becomes more concentrated in the amalgam. This method involves the use of a mercury-lithium amalgam, which has a higher affinity for lithium-6. When the amalgam is added to solutions containing lithium hydroxide, lithium-6 preferentially forms a complex with the amalgam, allowing for its separation from lithium-7. COLEX separation has been the go-to method for large-scale lithium enrichment due to its technical efficiency and cost-effectiveness. However, it comes with significant drawbacks, including the use of toxic mercury, the formation of hazardous waste, and high energy consumption. These factors pose environmental concerns and cleanup challenges. Nevertheless, ongoing research aims to develop cleaner alternatives for lithium enrichment while mitigating the risks associated with COLEX separation. As of 2023, there are no industrial-scale facilities capable of fulfilling the future demands of commercial fusion power plants.
-
Laser processes on metal vapor involves the use of lasers to selectively excite and vaporize lithium atoms, separating lithium-6 from lithium-7 based on their differing vaporization properties.
-
Crown-ether separation: Crown ethers are special molecules that act like tiny "crowns" made of oxygen atoms. These crowns have a hollow center that can trap or hold other smaller molecules, like a king or queen wearing a crown. Crown ethers are good at grabbing certain ions or molecules and holding onto them tightly, which makes them useful in the extraction or separation of substances. In chemistry language, crown ethers are cyclic compounds with a structure that allows them to selectively bind certain metal ions. By utilizing specific crown ethers, lithium-6 can be selectively complexed and separated from lithium-7.
-
Centrifugal extraction is the process where a gaseous compound of lithium, such as lithium chloride (LiCl), is converted into a vapor and then introduced into a centrifuge. The centrifuge spins at high speeds, causing the isotopes to separate based on their mass differences. Since lithium-6 is slightly lighter than lithium-7, it tends to concentrate towards the center of the centrifuge, while lithium-7 accumulates towards the outer region. This separation can be achieved by collecting the lithium-6-enriched fraction from the center of the centrifuge.
Read the Lithium Isotope Separation: A Review of Possible Techniques paper for more techniques and their history.
Alright, now that we've explored a few methods for separating lithium-6, let's pause for a moment before we consider a company to separate lithium. Understanding the history is key – we need to know what has been attempted before and, even more importantly, what was once deemed impossible but might just be within our reach today.
Source and continue reading.
The History of Lithium-6
Back in the days of the Cold War, when things were tense and nuclear bombs were on people's minds, a mission to separate lithium isotopes took center stage. They needed the famous lithium-6 for the powerful thermonuclear weapons. The United States had a top-secret operation going on at the Y-12 National Security Complex in Oak Ridge, Tennessee. They tested out three different processes there: OREX (organic exchange), ELEX (electrical exchange), and COLEX (column exchange). Not much is known about the details, given the classified nature of the work. But over time, some information has been declassified, shedding light on what went down back then.
Apparently, COLEX was the star of the show, proving to be the most efficient process. They enriched a whopping 442 tons of lithium hydroxide between 1952 and 1963. Now, we still have some of that stuff stored in the Department of Energy's facilities in Oak Ridge and Portsmouth, Ohio.
Now, let's talk about the dark side of the COLEX process (and its partner, ELEX). These methods had a bit of an environmental issue. You see, they involved using mercury, which can be a bit tricky to handle. The thing is, back in the day, they weren't fully aware of the potential dangers and didn't have the same safety standards we have now. They simply didn’t know much about mercury’s chemistry. So, hundreds of tons of mercury ended up getting lost in waste streams or just evaporating into the air. Not the best situation, I know. But hey, you live and learn, right? We've come a long way in understanding the chemistry of mercury and the importance of protecting the environment. Back then, though, it was a bit of a mess.
Fast forward to the present day. As far as people can tell, the demand for lithium-6 on the global market is quite limited. The black market might be a different story as anyone making nuclear weapons will need lithium-6, cough cough North Korea.
Anyways, most of the supply actually comes from the lithium-6 produced in Oak Ridge back in the day. So, the prices for lithium-6 on the free market, if you can find any, can be quite high, around $136 per gram (or $136,000 per kilogram). But get this, back in 1982, when the COLEX process was booming, the costs for enrichment were estimated to be around $1,000 per kilogram. Yeah, it's a pretty big difference. And you know why? It's mainly because the supply isn't keeping up with the potential demand. If more people start clamoring for lithium-6 in the future, those prices could skyrocket, or worse, the demand won’t be met, and fusion energy won’t happen.
Source and continue reading.
Trillion-Dollar-Idea: Separating Lithium-6
When possible, the best way to fix problems in the world is through the alignment of incentives via market mechanisms (aka the creation of a company).
So, let’s create a company to separate lithium-6 and here’s my business plan. Want to invest and help make this idea a reality? Send me an email and start this company.
Overview:
The rise of nuclear fusion holds immense potential for meeting our future energy needs. However, one critical challenge lies in the scarcity of lithium-6, a vital isotope required for fusion reactors. With 92% of naturally occurring lithium being lithium-7, obtaining a sufficient supply of lithium-6 becomes a pressing concern. This is where our groundbreaking business plan comes into play. We aim to establish an innovative and environmentally responsible lithium-6 separation facility that meets the future demands of commercial fusion power plants.
The Current Scenario:
Currently, the primary method for lithium-6 separation is the decades-old process known as COLEX (Column Exchange Separation), which relies on the use of mercury. Unfortunately, this method lacks significant innovation since its inception in the 1950s. Moreover, due to environmental and regulatory concerns associated with mercury, there is a pressing need for a revolutionary and sustainable solution. The lack of lithium-6 (counterargument in the next section) seriously threatens the success of future power plant applications of nuclear fusion.
Our Trillion Dollar Idea:
Our business centers around the innovation of lithium-6 separation, aiming to establish a cutting-edge facility that meets the future demands of nuclear fusion companies. By radically improving existing technologies and implementing state-of-the-art processes, we intend to disrupt the market and secure a monopoly on the supply of lithium-6.
Key Objectives:
- Develop a highly efficient, environmentally friendly, and sustainable method for lithium-6 separation, including effective mercury handling or a mercury-free alternative.
- Scale up the production capacity to meet the future requirements of commercial fusion power plants.
- Ensure strict compliance with regulatory standards and establish a reputation for responsible and sustainable practices.
Challenges and Solutions:
While this trillion-dollar opportunity is tempting, we acknowledge the challenges we must overcome. Dealing with large quantities of mercury poses environmental and operational concerns. Therefore, a significant focus of our business plan is to implement advanced mercury-safe or mercury-free separation technologies. By investing in research and development, we aim to create a safe and efficient process that aligns with stringent environmental regulations and ensures minimal waste generation. There are also signicant regulatory hurdles such as COLEX being banned in the United States. However, we believe we can learn from other defense contractors and work with the government to ensure compliance with all regulations.
Concluding Words: Our business plan for a lithium-6 separation facility stands at the forefront of the nuclear fusion revolution. By innovating and revolutionizing the existing COLEX process, we envision a future where we have a monopoly on the supply of lithium-6, serving as a critical partner for the burgeoning nuclear fusion industry. Together, let us take on this journey of technological innovation, sustainability, and unlocking the immense potential of nuclear fusion for a brighter, cleaner future.
"Fusion May Actually Work To Make Electricity"
Immediately after having this idea, I reached out to startups (and professors!) to test the market.
My first email was fired off to the CTO of Helion, one of the most promising fusion startups. I asked them about their concerns regarding the possible supply shortage of lithium-6.
This was their response:
For Helion, we are using a D-He3 fuel cycle so, we don’t need to breed tritium.
As we discussed before, the D-He3 reaction occurs when deuterium (an isotope of hydrogen) and helium-3 fuse. The reason some people believe the D-He3 reaction is the best path forward to generate clean energy is that all fusion energy is released in charged particles that can be directly converted to electricity.
The CTO of Helion then proceeded to quote Helion’s website:
Helium-3 is an ultra-rare isotope of helium that is difficult to find on Earth and used in quantum computing and critical medical imaging.
Helion produces helium-3 by fusing deuterium in its plasma accelerator utilizing a patented high-efficiency closed-fuel cycle.
Helium-3 has, historically, been very difficult to produce. Scientists have even discussed going to the Moon to mine helium-3 where it can be found in much higher abundance. Helion’s new process means we can produce helium-3 (no space travel required!)
Ok, Helion. Fair enough.
So, you don’t need lithium-6 but are you sure? We’ll see about that.
At the same time, I also sent an email to David Ruzic, one of the world's leading experts in nuclear energy, who happens to do consulting work with startups like Helium and others.
So, I asked him about the possible future lack of lithium-6. Here's what Professor Ruzic replied:
There are people working on experiments to do the separation to learn what is the most efficient process. Keep in mind that this is a nuclear process, so the amount of Li-6 you need to breed the tritium one would fuse is actually a very small quantity compared to mountains of coal, or tankers full of oil.
Also, fusion is at best still 15 years away from being on the grid. That is plenty of time for there to be a supply of Li-6.
Interestingly a real plant may not even want Li-6. Natural lithium is 95% Li-7 and 5% Li-6. Both of those will breed tritium, it is just that Li-6 has a higher cross-section. There are so many neutrons being produced, however, and the breeder is also a shield, so it may be large enough to breed enough tritium that the cross-section does not matter.
Wow, I was shown a different perspective and my mind was expanded. By now, I had received Helion’s reply so I told Professor Ruzic this:
Helion told me they're using a D-He3 fuel cycle they don't need to breed tritium and they get He3 from fusing deuterium in a plasma accelerator utilizing a high-efficiency closed-fuel cycle. Any thoughts on that?
To which Professor Ruzic replied:
I was just on the phone with Helion. Eventually, Helion may make a hot enough plasma to do D-He3, but that is far far ahead. Way before that they will have to do D-T like everyone else to prove the concept works.
Also, the easiest way to make He-3 is to make tritium and let it decay….. You don’t want to mine the moon for it!
The point is that enriching Li is likely not needed to breed enough tritium to make fusion energy. Also, breeding is not the most difficult challenge to solve.
After a few email exchanges, which by the way, all happened in a span of an hour, I couldn’t tell whether he was excited about the future so I asked him, “What are you personally excited about?”
Fusion may actually work to make electricity – I was never convinced I would see it in my lifetime earlier in my career. In the larger context though, Nuclear Fission works even better and is now extremely safe.
Gen IV reactors even burn up most of their long-lived high-level waste. To save the planet from CO2, we need to make much more electricity from nuclear sources instead of coal, oil, and natural gas.
"Fusion may actually work to make electricity." Well, that's exciting! Some people believe it might be achievable within this decade. That's definitely something to get excited about and be proud of as humans!!!
With this, we conclude our journey through nuclear physics. I hope you've learned a lot, found inspiration, and started contemplating the future of our world.
Abundant energy is on the horizon, and it's just a matter of time. My friends, that's the future we're heading towards.
But to get back to the future, let's first return to the present and dive into the world of batteries—from their history and chemistry to manufacturing and the quest for the ideal battery. Let's power you up
Lithium-Ion Batteries
If you've heard about lithium, chances are it's in the context of lithium-ion batteries, which power our everyday tools like phones. But what exactly are lithium-ion cells, and where does the term "cell" come from? And what about "ion"? Is it similar to the name "Ian," but with an "O"? Let's take it step by step.
So, first things first, let's understand the difference between lithium-ion cells and lithium-ion batteries.
A lithium-ion cell is essentially a single unit, a building block, of a lithium-ion battery. On the other hand, a lithium-ion battery is made up of multiple lithium-ion cells connected together in specific configurations (either in series or parallel) to provide the desired voltage and capacity.
Lithium-ion batteries are a type of rechargeable battery that relies on lithium ions as the primary charge carriers. Rechargeable batteries? Yes, these are batteries that allow us to recharge and reuse them multiple times by supplying electrical energy, unlike single-use or disposable batteries. But what exactly is a battery? Essentially, it's a clever device that stores chemical energy and converts it cleverly into electrical energy, providing power to electronic devices. Imagine it as a special box that carefully holds energy inside, and when we connect it to phones and other devices, it generously supplies them with the power they need to function and move around!
I don’t know about you, but the fact that these batteries are rechargeable is pretty cool. It's fascinating to think about how they can be used over and over again. But how does that work? How is it even possible for batteries to be recharged?
Rechargeable batteries work through reverse chemical reactions that occur during charging and discharging. When a rechargeable battery is connected to an external power source, such as an electrical outlet, the electrical energy is used to undo the chemical reactions that took place during discharge. This restoration process replenishes the battery's energy storage capacity, making it ready for use again.
Hold a second and pause for a moment.
Chemical reactions happening inside your phone???? That’s new! In simple terms, here's a fun explanation: inside the battery, there are tiny particles called lithium ions that love to dance around, creating electricity. As they dance, the battery gradually loses its ability to produce electricity. But don't worry, there's good news! When you recharge the battery, those dancing particles move back to where they started, and the party begins again. This means the battery becomes ready to produce electricity again, keeping your phone powered up and ready for action!
Later, we'll explore a more detailed explanation of these chemical reactions that make our devices work.
Batteries Explained
We need to know what a battery actually is – it's like a special box that stores energy and gives power to things. To grasp how batteries work, we'll explore the key components that make them function, such as electrodes, cathode, anode, and electrolyte. Don't be intimidated by these fancy-sounding terms; they're just names that describe what something is or how it works. Let's start learning without fear!
Electrodes are crucial components inside a battery that have roles in storing and releasing electrical energy. Simply put, an electrode is a thing that carries electricity (aka an electrical conductor). Typically, electrodes are made of metals connected to something that is not metal. Once we grasp the simplicity of what an electrode is, we can get into the two types of electrodes found in a battery: the cathode and the anode.
The cathode in a battery is the “lithium host,” welcoming and accommodating lithium ions as they check in and check out. These lithium ions are like guests that carry electrical charges. When the battery is being charged, lithium ions move from the cathode through a substance called an electrolyte to the anode. The cathode attracts and holds onto the lithium ions during this charging process. In essence, the cathode's composition includes a material that contains lithium. [4]
While the anode serves as the "lithium parking lot," where the lithium ions park when the battery is being charged, it's constructed using a different material than the cathode, typically graphite. As you use the battery during the discharge process, the lithium ions depart from the anode and journey back to the cathode through the electrolyte. This movement of lithium ions generates an electric current that powers your device. It's like a constant cycle of lithium ions shuttling back and forth between the anode and cathode, delivering the energy needed to keep your device running smoothly.
What about the electrolyte? It’s a special liquid or gel-like substance that enables lithium ions to travel between the cathode and anode while ensuring they never make direct contact. It acts like a bridge, providing a pathway for the lithium ions to move through. Additionally, the electrolyte is in charge of maintaining the battery's safety and stability during its operation.
The curious reader will ask, “Juan David, what's the difference between the electrolyte in a battery and the one in Gatorade?"
They share the same name but serve distinct purposes and have different compositions. In a lithium-ion battery, the electrolyte is a chemical substance that facilitates the flow of lithium ions between the cathode and anode. It typically consists of a dissolved lithium salt helping the smooth movement of lithium ions, while also preventing reactions that could harm the battery's performance or safety.
On the other hand, the electrolyte in Gatorade and similar drinks is a mixture of various ions like sodium, potassium, and chloride, along with sugars and flavorings. In this context, electrolytes help maintain a proper balance of ions in the body and promote proper hydration. They play a critical role in replenishing essential minerals lost through sweating and support vital bodily functions, such as nerve signaling and muscle contractions.
Both electrolytes are called the same but they have distinct roles—one being the "facilitator" of lithium-ion movement in batteries, and the other being the "replenisher" of essential minerals in our bodies.
Now, returning to our discussion of batteries.
In a lithium-ion battery, cathodes, and anodes are constructed with different materials to serve their specific purposes. The cathode serves as the electrode where positive charges, in the form of lithium ions, are received and stored during the charging process. It typically consists of a lithium-rich material, containing a substantial amount of lithium. This design allows the cathode to have a high storage capacity for lithium ions.
On the other hand, the anode is the electrode where the negative charge (electrons) is released and received during the discharging process. The anode is usually made of a material that can intercalate (absorb and release) lithium ions, such as graphite or silicon. This allows the anode to store and release the lithium ions as the battery charges and discharges.
By using different materials for the cathode and anode, the battery can efficiently store and release lithium ions throughout the charging and discharging cycles. This optimization ensures the battery's reliable performance, energy efficiency, and long-term stability.
The flow of lithium ions generates an electric current that serves as the power source for your devices.
But have you ever wondered what happens after the electrical current is generated?
Electrical current is like the flow of water, as it involves the movement of electrons through a conductor, such as a wire. The more electrons that flow, the stronger the current.
A battery is like a water pump, creating an electrical pressure difference, just like the pressure in a pipe. This difference in electrical potential acts like a force that pushes electrons through a wire, kind of like water flowing through the pipe. The amount of current flowing through the wire depends on the battery's voltage and the wire's resistance. Think of voltage as the pump's power and resistance as how narrow or wide the pipe is. Ohm's Law (V=IR) helps us understand this relationship.
Now, electrical current is a big deal because it's what powers all sorts of devices, from our lights and appliances to our high-tech computers. It's also essential for transmitting energy over long distances.
Let's get a clearer picture of how electrical current works. Imagine a line of people standing close together, and each person in the line represents an electron. Now, when someone at the front gives a little push to the person next to them, that push gets passed down the line, eventually reaching the person at the back. It's like a chain reaction of pushes!
This analogy helps us understand how electrical current flows. In a battery, the electrons are pushed by the battery's power, and they, in turn, push each other along the wire, creating the flow of electricity. It's like a team effort where everyone contributes to keep the current flowing.
We've covered some important components and concepts that play a key role in how batteries work.
But you know what's really fascinating? Batteries have a history of their own so let’s go there!
The History of the Battery
Every invention is a notable example of our human ingenuity, and the battery is no exception. Sparks of brilliance ignited the path toward controlling electricity.
Long ago, in the ancient lands of Mesopotamia, the Babylonians discovered a curious artifact, known as the Baghdad Battery. This enigmatic device, dating back to the 3rd century BCE, consisted of a clay jar, an iron rod, and a copper cylinder. Like a cryptic puzzle piece, it hinted at the possibility of storing electrical energy. [5]
Centuries later, in the 18th century, an Italian scientist started the electrical revolution by creating the voltaic pile. This thing was like a tower of power, this stack of copper and zinc plates, separated by brine-soaked paper disks, produced a steady current, unlocking the potential of portable energy.
As the world marveled at the voltaic pile, another brilliant mind, Benjamin Franklin, coined the term "battery" in 1749 during his electric experiments. Inspired by the military's synchronized weapons, Franklin linked Leyden jar capacitors, multiplying their power and charge. His ingenuity gave birth to the concept of combining multiple vessels to unleash a stronger electric force.
Yet, in the early stages, batteries were far from perfect. Their voltages fluctuated, and their current output was insufficient for sustained use. It was not until 1836 that British chemist John Frederic Daniell created the practical and reliable Daniell cell. This remarkable invention, a copper pot filled with a copper sulfate solution, housed an unglazed earthenware container immersed in sulfuric acid and a zinc electrode. The Daniell cell powered electrical telegraph networks, becoming an industry standard and a beacon of progress.
However, the Daniell cell and other early batteries faced limitations. They relied on liquid electrolytes, prone to leakage and spillage, making them unsuitable for portable devices. Fragility and potential danger lurked within their glass jars, hindering their usability. The world yearned for a new solution.
In the late nineteenth century, the dry cell battery illuminated the way forward and the battery’s potential truly surged akin to a lightning bolt across the sky. With its paste-like electrolyte replacing the liquid counterpart, portable electrical devices became practical. This transformation ushered in a new era, enabling technology to break free from the confines of stationary power sources. The dry cell was a portable powerhouse that set the stage for the modern era. Its construction, like a layered cake, featured a zinc container, a carbon rod, and a moist paste of manganese dioxide and ammonium chloride. This innovation birthed the iconic household battery, powering everything from flashlights to radios, igniting a new era of convenience and possibility.
Notably, vacuum tube devices of the past adopted a combination of wet and dry cells. The "A" battery, powering the filament, relied on a wet cell, while the "B" battery, providing plate voltage, embraced the dry cell. This marriage of battery types propelled advancements in electronics, igniting a symphony of sound and illumination.
As the decades marched on, the battery evolved with the ever-growing demands of technology. The birth of the lithium-ion battery in the 1990s ushered in a new era of mobility and versatility. Like a pocket-sized dynamo, it harnessed the power of lithium and danced with a lightweight design, fueling the rise of mobile phones, laptops, and electric vehicles.
With each passing year, new breakthroughs emerge, promising longer-lasting power, faster charging, and a greener footprint.
From the voltaic pile to the dry cell, the battery's journey has been one of innovation and progress.
The battery has powered our devices, illuminated our world, and brought us closer to the future. As technology continues to evolve, batteries will certainly continue to be in our lives for many years to come, especially the famous lithium-ion battery so let’s jump to another history lesson!
History of the Lithium-ion Battery
For years, a revolution in energy storage has been in the making. The stage was set for the grand entrance of the lithium-ion battery, a marvel of modern science and a catalyst for technological transformation.
But it wasn’t always like that, for many years, few people believed in lithium-ion batteries.
What changed? Belief, imagination, and lots of effort!
Picture a bowl of elements—lithium, cobalt, manganese—engaging in an intricate chemical tango. Like synchronized partners, they come together, creating an elegant symphony of energy storage. The year was 1970, and the stage was set for the birth of a game-changing innovation.
Stanley Whittingham, a visionary scientist, stepped onto the scene, pioneering the concept of intercalation—the art of wedging lithium ions between the layers of a material. It was a poetic union, a dance of lithium ions finding their perfect fit, nestled within the molecular embrace of titanium disulfide.
Whittingham introduced titanium disulfide (TiS2) as a cathode material, its layered structure providing a perfect sanctuary for lithium ions, unruffled by changes to its crystal structure. Whittingham's creation, the first functional lithium-ion battery, laid the foundation for a revolution yet to come.
Excitement filled the air as Whittingham's invention promised a new era of power. Exxon, eager to commercialize this breakthrough, stepped forward in the late 1970s. But they encountered hurdles along the way. The synthesis of TiS2 proved expensive and complex, like a delicate dance with moisture. As water met TiS2, toxic H2S gas was released, creating a formidable challenge. Moreover, the presence of metallic lithium in these batteries caused them to catch fire spontaneously. Like a fiery dragon, danger lurked within.
Undeterred, the quest for a safer alternative continued. In 1980, separate groups led by Ned A. Godshall and Koichi Mizushima, along with John B. Goodenough, sought to refine Whittingham's creation.
Goodenough, armed with his ingenuity, envisioned a new leading performer—a cathode made of cobalt oxide. This cathode, like a virtuoso violinist, produced a symphony of high voltage and energy density. After meticulous experimentation, they replaced TiS2 with lithium cobalt oxide (LiCoO2, or LCO), a layered structure offering higher voltage and enhanced stability. The stage was set for the first commercial Li-ion battery, yet the shadows of flammability still loomed.
Amidst this electrifying atmosphere, another luminary emerged. Rachid Yazami demonstrated the reversible electrochemical intercalation of lithium in graphite, like a beautiful dance between elements. He unveiled the lithium graphite electrode, a vital component in the Li-ion battery symphony.
But challenges remained.
The use of lithium metal anodes, while promising, posed safety risks with their instability and tendency to form dendrites.
The breakthrough came when an intercalation anode, mirroring the cathode's design, came to light. The shift marked a turning point, allowing the prevention of lithium metal formation during charging. It was the dawn of a new era.
In 1987, Akira Yoshino presented a revolutionary solution – a rechargeable lithium on battery with a "soft carbon" anode, resembling charcoal. The world embraced this innovation, and in 1991, Sony unveiled the first commercially available rechargeable Li-ion batteries. Toshiba and Asahi Kasei Co. soon followed suit, shaping a world powered by lithium.
The years that followed witnessed remarkable strides in energy density. Researchers replaced the soft carbon anode with hard carbon and later with graphite, an idea once deemed unfeasible but now driving progress forward.
As the twenty-first century unfolded, the Li-ion battery's star continued to rise. Production capacities soared, and the world witnessed the electrifying growth of this extraordinary power source.
The brilliance of those behind this groundbreaking invention did not go unnoticed. In 2012, John B. Goodenough, Rachid Yazami, and Akira Yoshino received the IEEE Medal for Environmental and Safety Technologies, a testament to their pioneering contributions.
And in 2019, the Nobel Prize in Chemistry celebrated Goodenough, Whittingham, and Yoshino for their transformative work on lithium-ion batteries.
But the story continues, with each chapter revealing new possibilities. New battery innovations continue to be created, producing higher and higher performances.
The lithium-ion battery stands as a symbol of innovation and hope. It has empowered our devices, transformed our lives, and illuminated a path toward a brighter, electrified tomorrow.
Honorary to John B. Goodenough
John Goodenough led a truly remarkable life and left an unforgettable impact on our world through his significant contributions. He not only served in WWII but also pursued a Ph.D. under the guidance of none other than Enrico Fermi.
Goodenough's journey is a shining example of an individual who followed his curiosity throughout his entire life until he passed away.
His legacy continues to inspire and shape the fields he touched.
A remarkable journey, a life well-lived, With contributions that forever give, From serving in the World War's strife, To unlocking the secrets of battery life.
A student of great minds, Enrico Fermi's mentee, John Goodenough, a scientific prodigy, In his prime, he helped create RAM, Pushing the boundaries of technology's exam.
But it was at 58, an age many would retire, That Goodenough's genius would truly inspire, For he invented the Lithium-Ion battery, A groundbreaking innovation, a game-changer in the battery's story.
With his brilliance, he harnessed energy's might, Creating a power source that burned bright, Revolutionizing portable electronics, electric cars, His invention reaching beyond the stars.
A lifetime of dedication, research, and toil, Goodenough's impact on science will forever uncoil, At 97, he became the oldest Nobel laureate ever, Recognition of his contributions that will falter never.
A century lived, a centennial celebration, John B. Goodenough, a beacon of inspiration, His legacy lives on, his spirit forever ablaze, A pioneer of science, illuminating our future's maze.
In gratitude, we honor this extraordinary man, Whose brilliance and perseverance forever span, John B. Goodenough, a true scientific luminary, May his legacy shine bright, an everlasting visionary.
What Happens After a Lithium-ion Battery is Connected to a Device?
What happens after the lithium-ion battery is connected to a device like a phone or a car and the energy in the battery is converted into electrical current to power the device?
- The battery, consisting of a cathode, anode, and electrolyte, is connected to the device.
- When a circuit is completed (the connection between the positive and negative terminals of the battery through a circuit, allowing the flow of electrons) by turning on the device, the chemical reactions within the battery start to occur.
- During the discharging process, lithium ions move from the anode to the cathode through the electrolyte. This creates an electric current.
- The electric current flows through the circuit, proving the necessary power for the device to function.
- Meanwhile, at the cathode, the stored lithium ions combine with electrons from the external circuit, completing the electrical circuit and maintaining charge balance.
- As the lithium ions move from the anode to the cathode, the anode gradually becomes depleted of lithium ions.
- Once the battery’s charge decreases to a certain level, indicating it’s discharged, it needs to be recharged by reconnecting it to a power source.
I was learning about this and was curious about how devices know when their batteries are low. How do devices know when the battery is low? Here's a quick overview:
- Battery sensors: Inside the divide, there are sensors that measure the voltage or current of the battery.
- Battery management system (BMS): The device’s operating system or firmware includes a battery management system that interacts with the battery sensors.
- Battery status indicator: The device’s user interface, such as the screen, may display a battery status percentage to indicate the remaining charge. This information is typically based on the data provided by the battery sensors and the calculations by the BMS.
Why The Lithium-Ion Battery?
You might have wondered, "Why did lithium-ion batteries become the most common and widely used?"
Lithium-ion batteries are super popular in consumer electronics like laptops, cell phones, and digital cameras, as well as in electric vehicles and other high-energy-demand applications. They stand out due to several advantages:
- High energy density: Lithium-ion batteries can store more energy (high energy density) in a given volume compared to other rechargeable batteries.
- Long life: These batteries can endure numerous charge and discharge cycles, making them last longer.
- Fast charging: They can be charged quickly, saving time and enhancing convenience.
- Low Self-Discharge: Lithium batteries exhibit a low self-discharge rate, meaning they retain their charge for longer periods when not in use. This makes them well-suited for applications that require long shelf life or intermittent use, as the battery does not drain significantly over time.
- High Voltage: Lithium-based chemistries offer a higher operating voltage compared to other battery technologies. This higher voltage allows for more efficient energy conversion and utilization, resulting in improved performance and power delivery.
- Environmental Considerations: Lithium is a relatively abundant element and has a lower environmental impact compared to some other battery chemistries, such as lead-acid batteries. Additionally, the shift towards renewable energy sources has increased the demand for energy storage solutions, and lithium-ion batteries have proven to be an effective and sustainable option in this regard.
But lithium-ion batteries are far from perfect with serious drawbacks:
- High cost: Lithium-ion batteries are more expensive than other rechargeable battery types.
- Safety concerns: Improper handling of lithium-ion batteries can pose safety risks.
- Memory effect: There is a "memory effect" concern, where the battery's charge-holding capacity may be reduced if not fully discharged before recharging.
These factors have collectively propelled lithium to its prominent position in battery technology. Ongoing research and development efforts continue to enhance lithium battery performance, safety, and cost-effectiveness, ensuring its continued dominance in the energy storage market.
While we're discussing common questions, it's an understatement to say that lithium-ion batteries can be dangerous. In fact, they have the potential to explode! But why is that the case?
Why Do Lithium Batteries Explode?
First off, lithium battery explosions are rare. It’s not like you can expect your phone to blow up at any moment. Unless, yeah, you know what I mean.
The primary reason behind safety risks for lithium batteries is what's known as "thermal runaway." This occurs when the battery heats up uncontrollably, triggering a chain reaction of chemical processes that generate even more heat. The situation becomes increasingly heated, leading to the release of flammable gases, potential breakage of the battery casing, and, in severe cases, an explosion or fire. These reactions involve various electromechanical processes, such as the decomposition of electrolyte components and reactions between electrode materials.
What can cause a thermal runaway? Overcharging, short-circuit (separator degrading), exposure to high-temperature or fire, or manufacturing defects.
Before you start freaking out. I mean, please don’t. Come on, you’re 100% reading this on a device powered by a lithium-ion battery, there are millions of lithium batteries that are safely used without incident so life is good, continue enjoying your lithium batteries devices!!!
Battery Manufacturing
Now that we understand how batteries work and the chemistry behind them, let's get into the manufacturing process.
The manufacturing process of lithium-ion batteries is complex and involves several steps. The process begins with the preparation of the electrode materials, followed by the assembly of the battery cell, and finally, the battery pack.
As batteries become more crucial in our lives, battery manufacturing will become even more important. Batteries are essential for any electronic devices including electric vehicles, portable electronics, renewable energy storage, and grid stabilization. If batteries don’t work, our world stops working.
What would we do without our phones, right? So, we need lots of batteries, and to make lots of batteries, we need to build manufacturing factories and make them work! Now, how does the manufacturing process actually work?
I like starting with an overview and start exploring the details. In this case, we will be reading a poem.
Poem on Battery Manufacturing
In the realm where electrons dance and flow, Where power is harnessed, a vibrant glow, Lies the realm of battery manufacturing, A symphony of science, ever captivating.
From the depths of mines, materials arise, Lithium, cobalt, and nickel's prize, Gathered with care, these elements bold, For a battery's tale, waiting to unfold.
In factories humming, with precision and grace, Electrodes take shape, in their designated space, Anodes and cathodes, meticulously prepared, Coated and dried, their destinies shared.
Mixing and blending, a harmonious blend, Active materials and binders, their journey must transcend, Homogeneity sought, with every stirring hand, To ensure performance, as batteries expand.
Coating machines spin, with steady grace, Slurries applied, in a delicate embrace, Metal foils adorned, with layers thin, Anode and cathode, their journey begins.
Slitting and cutting, with precision keen, Electrodes shaped, for the perfect scene, No burrs, no flaws, to disrupt the flow, A seamless path for currents to bestow.
The electrolyte, a mystical elixir, Facilitating ions, a magical mixer, Formulated with care, its components blend, Ensuring power and safety, till the end.
Cell assembly, a grand tapestry unfolds, Electrodes, separators, and electrolyte, it holds, Aligned with purpose, in pouches or rolls, The stage is set, as energy unrolls.
Welding terminals, a connection made, Positive and negative, a bond displayed, With precision and skill, their union secure, A symphony of currents, ready to endure.
Enclosing and sealing, a final embrace, Protecting the heart, with utmost grace, For batteries, like warriors, must stand tall, Ready to power, whenever we call.
Testing and assurance, a rigorous quest, Performance and safety, put to the test, Cycling and analysis, to measure their might, Batteries shining, in the realm of light.
From manufacturing's depths, a journey complete, Batteries crafted, a triumph so sweet, Empowering our world, with energy untamed, A testament to innovation, forever acclaimed.
So, let us marvel at the wonders they bring, From portable devices to electric wings, Battery manufacturing, a symphony divine, Powering our lives, through space and time.
Let's go for it and explore the manufacturing process in detail.
I. Battery Manufacturing Introduction
The battery manufacturing process involves several stages, from raw materials acquisition to final product assembly. Each step is decisive in ensuring the performance, quality, and safety of the batteries.
Battery manufacturing involves three main phases:
-
Electrode manufacturing: In this phase, the anode and cathode materials are mixed and then coated separately. The coated electrodes are then processed through various steps, including mixing, coating, rolling, slitting, sheet cutting, and die cutting. The electrodes, along with dried separators, are sent to the dry room for further processing.
-
Cell assembly: During this phase, the different components, including the anode, cathode, and separator, are assembled together to form the internal structure of a cell. Aluminum and copper tabs are welded on the cathode and anode current collector, respectively. Once the cell assembly is complete, the battery cells are packed and undergo testing.
-
Training, aging, and testing: This phase is crucial to ensure the right performance of the assembled battery cells. The cells undergo training, where they are charged and discharged multiple times to reach optimal performance. After training, the cells are aged to simulate real-world usage conditions. Finally, thorough testing is conducted to validate the performance and safety of the battery cells.
The entire manufacturing process can take anywhere from 6 to 18 weeks, depending on the availability of materials and the type of battery cell being produced. As batteries play an increasingly vital role in our lives, the efficiency and quality of battery manufacturing become more important than ever before.
II. Raw Materials Acquisition
Battery manufacturers procure crucial raw materials, including metals (such as lithium, cobalt, and nickel), chemicals, and electrolytes. These materials are typically sourced through mining operations or recycling processes.
Let’s explore each material in more detail
- Lithium: This highly reactive and flammable metal is mined and obtained for battery production in the form of lithium carbonate or lithium hydroxide.
- Nickel: An essential metal used in active cathode materials for modern batteries.
- Cobalt: Another key metal utilized in active cathode materials, contributing to battery performance.
- Manganese: In manganese-based Li-ion batteries, the cathode forms a spinel structure, creating a robust three-dimensional framework.
- Graphite: Serving as the anode material in lithium-ion batteries, graphite constitutes a significant volume proportion among all battery raw materials and significantly impacts cell production costs.
- Steel: Provides the best balance of strength, mass reduction, performance, cost, and environmental impact.
Next up, electrode production.
III. Electrode Manufacturing
Electrode manufacturing is an art as much as cooking is.
Imagine the production of electrodes for batteries as a culinary masterpiece, where each ingredient is carefully selected and combined to create a delectable final product. Let's explore this incredible process of electrode production and coating, crucial steps in the battery manufacturing journey.
First, the ingredients - the anode and cathode. The anode, storing, and releasing electrons, features a copper foil coated with graphite. The cathode, where positive ions reside during charging, consists of an aluminum foil coated with a specific chemistry like NMC (nickel-manganese-cobalt) or NCA (nickel-cobalt-aluminum). Just as a chef selects the finest ingredients, battery manufacturers choose these materials for their desirable properties.
Next, we move on to mixing the ingredients to achieve a homogeneous slurry, ensuring even distribution throughout the mixture. For the cathode, a blend of active material (e.g., NMC622), polymer binder (e.g., PVdF), solvent (e.g., NMP), and conductive additives (e.g., carbon) are carefully batch mixed. Meanwhile, the anode requires a mixture of active material (e.g., graphite or graphite + silicon), conductive material (e.g., carbon black), and polymer binder (e.g., carboxymethyl cellulose, CMC).
However, challenges arise during this process, such as controlling moisture content and maintaining particle integrity. Achieving the desired particle and pore size distribution is crucial, often requiring close collaboration with suppliers or innovative techniques like dual-layered approaches.
Coating comes next. Picture a skilled chef carefully applying a smooth, uniform layer of sauce on a dish. Similarly, the anode and cathode coatings are applied separately in a continuous coating process. The cathode, composed of a metal oxide for lithium-ion cells, is coated onto an aluminum electrode. The polymer binder acts as the "glue," adhering the anode and cathode coatings to the copper and aluminum electrodes, respectively. This coating step ensures the electrodes' stability and uniformity, just like the perfect finishing touch on a culinary masterpiece.
Immediately after coating, the electrodes undergo a crucial drying step. In a continuous process, infrared heating is used to remove the solvent from the coated foils. This not only ensures the electrodes' stability but also allows for the recovery of solvents, making the process more environmentally friendly. Challenges during drying include achieving homogeneity from the center to the edges of the electrodes, ensuring that all areas dry uniformly. Additionally, preventing cracking is another important consideration, as it can compromise the integrity of the electrodes.
Once the drying process is complete, the electrodes move on to calendering, which involves rolling them to achieve a controlled thickness and porosity. This step plays a significant role in optimizing the performance and overall structure of the electrodes. Controlling uniform thickness during calendering is crucial to ensure consistent performance across all battery cells.
Throughout the entire process, quality control is vital. Any foreign particles or impurities must be eliminated. Magnetic filters are often employed to remove metal particles, but they have limitations in capturing smaller particles. Additionally, the use of N-Methyl-2-pyrrolidone (NMP) as a solvent presents challenges due to its toxicity. Manufacturers conduct quality control tests downstream to ensure that the residual NMP is within acceptable limits, employing techniques like Gas Chromatography-Mass Spectrometry to analyze samples. It's like conducting a rigorous taste test to ensure the final dish is safe and delicious.
Just as a master chef masters the perfect balance of flavors and textures, battery manufacturers meticulously combine materials and coatings to create powerful and efficient energy storage devices that power our world.
IV. Cell Assembly
Cell assembly is the process of combining the anode, cathode, and separator to form the internal structure of a cell.
The process varies depending on the battery format, whether it's pouch, cylindrical, or prismatic cells. Automation and assembly techniques are used to ensure efficient and consistent cell production. Precise alignment and careful handling of components are essential to avoid short circuits or damage during the assembly.
Let's explore the steps involved in the cell assembly stage:
-
Slitting: Initially, electrodes are in standard widths of up to 1.5m and undergo slitting to match the final cell dimensions. Cutting the electrodes must be done carefully to avoid burrs on the edges, which could damage the separator and lead to short circuits. Insulation tape may be applied to prevent foil-to-foil shorts caused by burrs.
-
Drying: After slitting, the electrodes undergo further drying to remove all remaining solvent content and reduce free water to meet specifications. This ensures optimal electrode performance and prepares them for final assembly.
-
Shaping and Tagging: The electrodes are cut into their final shape, and tags are added for proper electrode identification and connection during assembly. Precise cutting is essential to ensure correct electrode shape, and preventing burrs or metallic particles is crucial.
-
Configuration: Depending on the cell format, electrodes are wound into a spiral for cylindrical cells, stacked for pouch cells, or adopt a flat-wound jelly roll configuration for some prismatic cells. Challenges include proper layer alignment, preventing separator punctures, and ensuring proper folding of the separator.
-
Welding: Anodes are connected to the negative terminal, and cathodes to the positive terminal. Welding the cell to busbars must be done carefully to avoid damage to internal welds. Challenges involve trimming tabs, aligning foils with tabs, and maintaining consistent energy bursts during welding.
-
Loading: In the final step, electrodes in rolls or stacked layers are loaded into cans or pouches, depending on the cell format. Challenges include debris avoidance, preventing damage to the jelly roll or stack, and maintaining sealing integrity. Pouch cell considerations include proper pouch cup formation, stack placement, and seal integrity.
Throughout the process, quality control is vital, conducting tests to verify the cell's integrity, and detecting any issues that may impact performance. As batteries become increasingly essential in our lives, battery manufacturing's meticulous processes ensure efficient, reliable, and safe energy storage for our modern world.
V. Cell Finishing
Cell finishing is the another step of battery manufacturing, where the cells are tested, sorted, and packaged for shipment.
During the cell finishing stage, critical steps are undertaken to prepare the cells for shipment and ensure their quality and performance.
-
Filling Stage: The dry cell is filled with electrolytes in this step. A partial vacuum is created to aid in distributing the electrolyte throughout all cell layers. Precise electrolyte dispensing based on a defined volume is ensured, and the cell's weight is checked before and after filling for accuracy. Challenges include maintaining environmental control, controlling injection pressure, and keeping low water content in the electrolyte for safety and battery health. Attention is given to traceability and proper wetting of all cell layers with electrolytes.
-
Formation and Sealing: The cell is charged during formation, leading to gas development. Before final sealing, the gases are released. The formation process, along with subsequent aging, take up to three weeks. Formation creates a solid-electrolyte interface (SEI) that protects the anode during fast charging and prevents irreversible electrolyte consumption. Challenges involve environmental control during charging, adhering to prescribed protocols, controlling degas evacuation pressure, and ensuring proper permanent cell sealing. Mass checks verify seal integrity.
-
Aging: Cells are stored at a controlled temperature for a specific period, allowing the SEI to stabilize, contributing to overall cell performance and longevity. Accelerating the forming and aging process without compromising quality, implementing fire detection systems, optimizing inventory, and ensuring safety through deoxygenated environments and nitrogen dowse are challenges faced in this step.
-
Comprehensive Control Checks: Prior to shipment, a series of comprehensive checks are conducted to ensure cells meet the required standards. These include analyzing charge/discharge cycle data, assessing delta OCV rate, conducting cell trimming, checking mass and dimensions, and performing leak and thickness checks for pouch cells. Visual inspections identify surface, tab, or seal anomalies. All data is recorded for traceability and quality assurance.
The cell finishing phase ensures the cells are in optimal condition and meet strict quality standards before reaching consumers. These meticulous steps contribute to the safe and efficient performance of battery-powered devices that have become indispensable in our daily lives.
VI. Battery Pack Integration
Battery pack integration is the step where the cells are assembled into modules and packs. This integration involves various steps to ensure seamless assembly and optimal performance of the battery pack.
-
Module Assembly: The first step in battery pack integration is module assembly. Battery cells are grouped together into modules designed to fit the specific requirements of the intended application. Modules may consist of a series or parallel arrangement of cells, depending on the desired voltage and capacity of the battery pack. Careful attention is given to the mechanical and electrical connections between cells to ensure reliable performance.
-
Thermal Management: Thermal management is critical for battery packs to maintain safe operating temperatures and prevent performance degradation. The heat generated during battery operation is efficiently dissipated using cooling systems such as liquid cooling or air cooling. Techniques like heat sinks, cooling plates, and thermal interface materials are employed to regulate temperature and avoid overheating.
-
Battery Management System (BMS): The BMS is an integral part of battery pack integration. It monitors and controls various parameters of the battery pack, including voltage, current, temperature, and state of charge. The BMS ensures the balanced operation of individual cells, optimizes charging and discharging processes, and provides protection against overcharging, over-discharging, and thermal runaway. Additionally, the BMS enables communication between the battery pack and external systems for monitoring and control purposes.
-
Enclosure and Safety Features: The battery pack is enclosed in a protective housing designed to meet safety standards and protect the battery from external impacts and environmental factors. The enclosure typically uses materials that provide structural integrity, electrical insulation, and resistance to fire and chemical exposure. Incorporating safety features like fuses, circuit breakers, and insulation barriers helps prevent electrical faults and mitigate potential hazards.
Battery pack integration plays a crucial role in ensuring the reliability, safety, and overall performance of battery-powered devices and electric vehicles. By carefully orchestrating these steps, manufacturers create battery packs that are ready to power our world efficiently and securely.
VII. Final Product Assembly and Packaging
The final product assembly and packaging stage involves integrating the battery packs into end products, such as electric vehicles, consumer electronics, or energy storage systems. This stage focuses on ensuring proper installation, connection, and testing of the battery packs to meet the specific requirements of the intended applications.
-
Mechanical Integration: This step involves physically installing the battery pack into the product. It may include designing custom mounting brackets, securing the battery pack in the designated space, and ensuring proper alignment and fit within the product's overall structure. Mechanical considerations also involve integrating electrical connectors, terminals, and busbars for seamless electrical connections.
-
Electrical Integration: In this phase, the battery pack is connected to the product's electrical system. It includes establishing necessary electrical interfaces, such as power input/output connections and communication interfaces with the product's control systems. Proper electrical routing, insulation, and shielding techniques are employed to ensure reliable and efficient electrical integration.
-
Testing and Quality Assurance: Thorough testing and quality assurance procedures are conducted to verify the functionality, performance, and safety of the final product. This includes functional testing of the battery pack, as well as testing the integration between the battery pack and the product. Various tests, such as charge-discharge cycles, capacity measurements, and safety tests, are performed to ensure compliance with industry standards and customer requirements.
-
Packaging and Labeling: Packaging and labeling play a vital role in protecting the final product during transportation, storage, and display. The packaging is designed to provide adequate cushioning, shock absorption, and protection against environmental factors. Proper labeling is essential for product identification, regulatory compliance, and conveying important safety information to end-users.
This final stage ensures that the battery-powered end products are fully operational, safe, and ready to serve their intended purposes effectively. By meticulous integration, testing, and packaging, manufacturers deliver high-quality products to consumers, enhancing their daily lives and driving technological advancements forward.
VIII. Distribution and Supply Chain Management
Distribution and supply chain management encompass the logistics, transportation, and coordination of battery products from manufacturing facilities to end-users. Effectively managing the distribution and supply chain is crucial to ensuring timely delivery, optimizing inventory levels, and meeting customer demand.
-
Inventory Management: Inventory management involves tracking and controlling the stock of battery products throughout the supply chain. This includes managing raw materials, work-in-progress (WIP), and finished goods inventory. Effective inventory management strategies ensure optimal stock levels, minimize excess inventory, and prevent stockouts to meet customer demands while minimizing carrying costs.
-
Logistics and Transportation: Efficient logistics and transportation play a pivotal role in distributing battery products. This includes selecting appropriate transportation modes, optimizing routes, managing customs and regulatory requirements, and coordinating with logistics partners. Timely and reliable transportation ensures that battery products reach their destinations safely and on schedule.
-
Warehousing and Storage: Warehousing and storage facilities are essential components of the supply chain for battery products. These facilities provide secure storage, proper handling, and inventory control to prevent damage, theft, or degradation of the products. Warehouse management systems (WMS) and inventory tracking technologies enable efficient storage and retrieval of battery products.
-
Reverse Logistics and After-Sales Service: Reverse logistics involves managing the return and recycling of battery products, as well as providing after-sales services. This includes processes for product returns, recycling or disposal of end-of-life batteries, and addressing customer inquiries, warranty claims, or repairs. An effective reverse logistics system ensures proper handling of used batteries and promotes sustainable practices.
Battery manufacturers ensure a smooth flow of products from production to the hands of consumers to contribute to the seamless operation of industries that rely on battery technology.
IX. Battery Recycling and End-of-Life Management
Recycling and end-of-life management of batteries is essential to minimize environmental impact, conserve valuable resources, and ensure the responsible disposal of hazardous materials. By following proper recycling and disposal processes, we can recover valuable materials and prevent pollution, contributing to a cleaner and more sustainable future.
Efficient collection and sorting systems are put in place to gather used batteries for recycling. Collection points, drop-off centers, and dedicated recycling programs are established to encourage consumers and businesses to dispose of batteries properly. Sorting processes are employed to categorize batteries based on their chemistry and design, as different types of batteries require specific recycling methods.
Battery recycling technologies are then utilized to extract valuable materials from used batteries. These technologies vary depending on the battery chemistry and may include processes such as mechanical shredding, chemical leaching, pyrometallurgical, hydrometallurgical methods, and refining techniques. Through these processes, we can recover metals like lithium, cobalt, nickel, and other valuable materials from the batteries, preventing these resources from going to waste.
Since batteries contain hazardous materials that can harm the environment if not handled properly, hazardous waste management practices are crucial. This ensures that toxic substances, such as heavy metals and corrosive chemicals, are managed safely and in compliance with regulations. Proper storage, transport, treatment, and disposal of hazardous battery components are essential steps in this process.
Environmental regulations and compliance standards set guidelines for the proper handling, recycling, and disposal of batteries to protect human health and the environment. By complying with these regulations, we ensure that all recycling and disposal processes are conducted responsibly and sustainably.
In our quest to reduce reliance on the countries that dominate raw material production, the establishment of a comprehensive recycling infrastructure becomes increasingly crucial. While processes for recovering materials from small lithium-ion batteries, like those found in cell phones, are already underway, the recycling of larger and more powerful vehicle batteries poses greater challenges.
Umicore is leading the way in commercial battery recycling. Their innovative process involves two distinct phases: pyro-metallurgical and hydro-metallurgical. Through thermal processing, they generate an alloy containing cobalt, nickel, and copper, as well as a slag fraction. The subsequent hydro-metallurgical stage allows for the recovery of these metals. Umicore's recycling plant currently has a capacity of 7,000 tons of battery mass annually, equivalent to around 35,000 electric-vehicle batteries.
Another notable endeavor is Volkswagen's pilot plant for recycling high-voltage vehicle batteries in Salzgitter, Germany. This plant aims to recover 100% of lithium, nickel, manganese, and cobalt, along with 90% of aluminum, copper, and plastic. Operating at a capacity of recycling 3,600 battery systems per year (approximately 1,500 tons of battery mass), and the plant is designed for scalability as more used batteries become available. Importantly, Volkswagen's recycling process avoids energy-intensive smelting in a blast furnace. The used battery systems are deep discharged, disassembled, and shredded to form granulate. This granulate is then dried, resulting in the production of aluminum, copper, plastics, and a black powdery mixture rich in essential battery raw materials: lithium, nickel, manganese, cobalt, and graphite. [6]
X. Conclusion
Battery manufacturing is no simple task, let me tell you. It's a whole intricate process that requires attention to every tiny detail and sticking to strict standards. From the very beginning, when they gather the raw materials, all the way to putting the final product together, every step is crucial to meet the increasing demands of our world.
And you know what's awesome? Throughout this whole process, they're super mindful of the environment. They're all about thesustainable sourcing of raw materials, finding ways to use energy efficiently, and making sure they handle waste responsibly. They're always on the lookout for new ways to reduce their impact, like using recycled materials and trying out greener techniques.
Quality control is a big deal in battery making. They test those babies at every stage to make sure they perform like champs and, more importantly, don't blow up in our faces. They put them through rigorous testing, like charge-discharge cycling and impedance analysis, to meet industry standards and keep us safe.
Oh, and safety? Top priority! They're serious about preventing any accidents. From the formula for their electrolytes to the way they put the cells together, they follow strict protocols to avoid any nasty stuff, like short circuits or overheating. They've got these cool battery management systems (BMS) that keep a close eye on the batteries, making sure they don't overcharge, over-discharge, or turn into mini fireballs.
And guess what? Even when batteries reach the end of their lives, the story's not over. They don't just toss them away like yesterday's trash. They recycle them and dispose of them responsibly. That way, they recover valuable materials and keep our environment clean and happy.
Battery manufacturing is a mix of science, technology, and being green. These manufacturers are always looking for ways to improve and be more sustainable as the demand for batteries keeps skyrocketing. They're paving the way for a future that's cleaner, greener, and more energy-efficient. Can you say "wow"?
Just like the way batteries power our devices, the manufacturing process itself is what empowers us with reliable energy storage. Each step is a building block, and they all fit together perfectly to make the magic happen. They integrate all these components, and run a bunch of tests, and make sure everything is safe for us and Mother Earth.
So, next time you pick up your phone, hop in an electric car, or use renewable energy storage, take a moment to appreciate the journey those batteries took to get there. It's not just some boring process; it's the energy that's driving us toward a future that's electrifying and sustainable. Battery manufacturing is the real hero here, making our lives better one charge at a time.
We started with a poem so we have to end with another poem:
In the realm of batteries, the future shines bright, With sparks of innovation, a dazzling sight. From manufacturing's realm, they emerge with glee, Powering our world, setting us all free.
With each passing day, their potential expands, Electric dreams taking hold in our hands. From smartphones to cars, a vibrant parade, A future powered by batteries, never to fade.
No more tangled cords or running out of juice, Batteries bring convenience, oh what a truce! They'll power our homes and cities, with clean energy, A sustainable world, oh how thrilling it will be!
So let's embrace this future, with excitement and cheer, For batteries will light up the path, crystal clear. A world powered by innovation and might, With batteries leading the way, oh what a sight!
The future is bright, with batteries at the helm, Unleashing possibilities, breaking the realm. So let's embark on this journey, with a joyful tune, A future powered by batteries, under a bright moon!
Goodbye, battery manufacturing!
Differences Between Car and Phone Battery Manufacturing?
Is there a difference between car and phone battery manufacturing? Yes, there is. The main difference is the size and scale of production. Car batteries are larger and require more energy capacity and power delivery. Phone batteries are smaller and require less energy capacity and power delivery.
For your reference, here's a table that summarizes the differences between car and phone battery manufacturing:
| Aspect | Car Battery Manufacturing | Phone Battery Manufacturing | |-----------------------------|----------------------------------------------------------|--------------------------------------------------------| | Size and Scale | Larger scale production due to the need for higher energy capacity and power delivery. | Smaller scale production due to the compact size of phones and limited energy requirements. | | Battery Type | Traction batteries designed for electric vehicles, often Lithium-Ion (Li-Ion) or Nickel-Metal Hydride (Ni-MH). | Lithium-Ion (Li-Ion) batteries commonly used for phones. | | Energy Capacity | Higher energy capacity to meet the demands of electric vehicles and provide long-range driving. | Lower energy capacity to meet the power needs of smartphones and ensure portability. | | Charging Speed | Focus on fast charging capabilities to reduce charging time and improve the convenience for car owners. | Focus on optimizing battery longevity by implementing slower charging methods for phone batteries. | | Electrode Stacking | Thicker electrode stacking due to larger cell sizes and the need for higher energy density. | Thin electrode stacking to accommodate the slim form factor of phones while maintaining efficiency. | | Heat Dissipation | Advanced heat dissipation methods to manage heat generated during fast charging and prolonged usage. | Focus on efficient heat dissipation to prevent overheating during phone usage and charging. | | Production Volume | Moderate to high production volume to keep up with the demand for electric vehicles worldwide. | Extremely high production volume to cater to the global smartphone market and constant upgrades. | | Automation | Extensive automation to ensure efficient production and maintain consistency in battery quality. | Automation implemented for precise assembly in a controlled environment to meet strict standards. | | Safety Measures | Stringent safety measures to prevent thermal runaway and ensure the safety of electric vehicle passengers. | Comprehensive safety measures to prevent battery-related incidents and ensure user safety. | | End-of-Life Management | Recycling and responsible disposal practices for used electric vehicle batteries to recover valuable materials. | Recycling and disposal practices for used phone batteries to minimize environmental impact. | | Environmental Impact | Environmental considerations focus on minimizing emissions during production and promoting sustainable materials. | Emphasis on reducing the ecological footprint through efficient use of materials and recycling. |
Please note that the table provides a general comparison and that the specifics of car and phone battery manufacturing may vary depending on the manufacturer and the technology used.
Alternatives to Lithium-Ion Batteries
While lithium-ion batteries have become the dominant technology in the battery industry due to their proven performance, mature manufacturing processes, and cost-effectiveness. However, as advancements continue to be made in alternative energy storage technologies, we may see a more diverse landscape in the future, with a range of options that cater to specific application requirements and sustainability goals.
Let's explore these alternatives and the ongoing research and development efforts:
-
Sodium-Ion Batteries: Operating on a similar principle to lithium-ion batteries, sodium-ion batteries use sodium ions for energy storage. With sodium being more abundant and less expensive than lithium, these batteries offer a potential cost-effective solution. However, they are still in early development, and further research is needed to improve their energy density and cycle life.
-
Solid-State Batteries: Utilizing solid electrolytes, solid-state batteries offer advantages like higher energy density, improved safety, and faster charging times compared to traditional lithium-ion batteries. They have the potential to overcome limitations such as thermal runaway and capacity degradation. However, commercialization and scalability are still under active research.
-
Fuel Cells: Fuel cells convert the chemical energy of a fuel, like hydrogen, directly into electrical energy through electrochemical reactions. They offer high energy efficiency and zero emissions, making them suitable for various applications. Challenges like hydrogen storage and infrastructure development need to be addressed for widespread adoption.
-
Flow Batteries: Flow batteries store energy in liquid electrolyte solutions in external tanks, allowing flexible scalability for large-scale energy storage applications. Vanadium redox flow batteries are well-known, but other chemistries are being explored for grid-level storage.
In addition to the alternatives mentioned, there are other notable developments:
-
Lithium-Sulfur Batteries: Promising higher energy density, these batteries use sulfur as the cathode material, which is abundant, low-cost, and environmentally friendly. Overcoming challenges in cycle life is a focus of ongoing research. These batteries face challenges such as the dissolution of sulfur and the formation of undesirable byproducts, which can limit their cycle life. Researchers are actively exploring solutions to overcome these obstacles and improve the performance and stability.
-
Solid-State Lithium-Metal Batteries: These batteries aim to replace the traditional graphite anode with a solid-state lithium-metal anode, offering even higher energy density and improved safety. However, developing stable solid-state electrolytes and addressing dendrite formation on the lithium-metal anode remain key challenges in realizing the full potential of this technology.
-
Magnesium Batteries: Investigated as an alternative to lithium-ion batteries, magnesium-based batteries have the potential for higher energy density. However, magnesium-ion mobility is currently slower than lithium-ion mobility, leading to lower battery performance. Researchers are exploring various strategies to enhance magnesium-ion conductivity and overcome other technical hurdles in order to commercialize magnesium batteries.
-
Lithium-Air Batteries: These batteries are also known as the lithium-oxygen battery. These rechargeable batteries use oxygen from the air to react with lithium ions, releasing a tremendous amount of energy during discharge and providing incredibly high energy density. The key innovation lies in the cathode, where oxygen from the surrounding air reacts with lithium ions and electrons to form lithium oxide (Li2O) during discharge. Overcoming stability and sealing challenges is essential for widespread adoption.
-
Beyond Batteries: There is growing interest in alternative energy storage solutions beyond conventional batteries. These include supercapacitors, which offer rapid charge and discharge rates but lower energy density, and mechanical energy storage systems such as flywheels and compressed air energy storage.
What will the future hold? No one knows for sure. Lithium-ion is winning the race for now, but the battery industry is constantly evolving, and we may see a new winner in the future. Only time will tell. Let's keep adapting, may innovation continue to thrive, and may the best technology win!
The Ideal Battery
If you're an entrepreneur or just someone curious about tackling important problems, let me tell you about the ideal battery the world is looking for. If we can get there with some innovation or solutions, it'll have a huge impact on our world and even make you a ton of money.
What does this perfect battery look like?
Imagine a battery that's super lightweight, but can store an enormous amount of energy. It would charge up crazy fast and last for a really long time before needing to be recharged. Plus, it would be way more environmentally friendly, reducing our reliance on non-renewable resources.
Having a battery like that would revolutionize so many industries. Think about electric vehicles that can travel longer distances without constant recharging, or renewable energy systems that can store excess power for later use. And of course, it would be a goldmine for anyone who can come up with the breakthrough technology to make it happen.
-
High Energy Density: The battery should be able to store a large amount of energy in a compact and lightweight form, allowing for extended use without frequent recharging.
-
Rapid Charging: Quick charging capabilities are essential for convenience and efficiency, reducing downtime for devices and vehicles.
-
Long Cycle Life: A durable battery with a long lifespan would minimize the need for frequent replacements, reducing waste and resource consumption.
-
Enhanced Safety: Safety is paramount, and the ideal battery would be highly resistant to overheating, fires, or explosions, ensuring user confidence and trust.
-
Fast Discharge Rate: The ability to deliver energy rapidly and consistently is crucial for applications that demand high power output.
-
Cost-Effectiveness: An affordable battery solution would make it accessible to a broader range of industries and consumers, driving widespread adoption.
-
Environmental Friendliness: A battery that relies on sustainable materials and eco-friendly manufacturing processes would reduce its environmental impact.
-
Versatility: The ideal battery should be compatible with various applications, from small electronic devices to electric vehicles and renewable energy storage.
-
High Efficiency: A battery with minimal energy loss during charging and discharging would maximize its performance and overall energy utilization.
-
Scalability: For large-scale applications, such as grid-level energy storage, the battery should be easily scalable without compromising its performance.
-
Specific-use cases: Instead of trying to create a one-size-fits-all battery, consider focusing on making the absolute best phone or car battery ever. Aim for a battery that outperforms current solutions by ten times! By honing in on these specific applications, you increase your chances of success compared to trying to develop a universal battery.
-
Manufacturing: Making a prototype is one thing, but how do we scale up production to manufacture not just one, but millions, or even billions of these exceptional batteries?
-
Something else: Don't limit yourself to just batteries. Who knows, you might stumble upon an entirely different technology or innovation that meets the requirements. The key is to fulfill the specified criteria, and how you achieve it is entirely up to you. Whether it's a battery breakthrough or an alternative solution, as long as it meets the desired performance levels, it's worth exploring.
So, if you're up for the challenge and want to make a real difference, go ahead and work on this dream battery. Not only will it help our world by cutting down on emissions and pollution, but it'll also be a major win for your own success and prosperity. The opportunities are endless, and I can't wait to see what incredible ideas and solutions come from them.
Is Lithium Abundant?
With so much talk about lithium batteries, how abundant is this thing?
From Wikipedia:
Another 2011 study at the University of Michigan and Ford Motor Company found enough resources to support global demand until 2100, including the lithium required for the potential widespread transportation use. The study estimated global reserves at 39 million tons, and total demand for lithium during the 90-year period annualized at 12–20 million tons, depending on the scenarios regarding economic growth and recycling rates.
Lithium is as abundant as glitter in a preschool art class. It's scattered everywhere, shimmering and twinkling in vast quantities. You can't escape its sparkling presence, just like you can't escape the urge to make arts and crafts with glitter. So, when it comes to lithium, we've got plenty to go around and power our devices without worry!
Why is it abundant? Its origin.
Lithium is relatively abundant because of its origin and the processes that occur within stars. It is produced through stellar nucleosynthesis, specifically during the fusion reactions that take place in stars. These reactions generate heavier elements like lithium from lighter elements like hydrogen and helium. Over billions of years, as stars go through their life cycles and eventually explode in supernovae, they release the elements they've synthesized into the surrounding space. This ejected material then becomes part of interstellar clouds, which eventually collapse to form new stars and planetary systems, including our own. Thus, the abundance of lithium in the universe can be attributed to the natural processes of stellar evolution and the recycling of stellar material over vast cosmic timescales.
Lithium is the 25th most abundant element in Earth's crust, with approximately 20 milligrams of lithium per kilogram of crust. [7]
So we’re not running out of Lithium any time soon! However, as demand increases for Lithium, what we’ll need will be innovation in lithium extraction and refining technology to ensure a stable and lasting supply of lithium. And sure sustainable as well.
Some people have concerned themselves about geopolitical wars in the future from the future. But look, I highly doubt this. If lithium is indeed needed, you know what will happen? We will simply discover new extraction methods, new sources of Lithium, or we will simply use something else.
Scarcity often means a lack of technological progress as Malthus learned the hard way. Technology is the way out of our problems (and sometimes more problems but for that, we will need to solve the Unabomber manifesto.
Talking about technological progress, you want to know where there’s a truckload of lithium? THE OCEAN. But it’s tricky. The concentration is extremely low, 0.17 mg·L–1, which is about 1 part per million (ppm). This means that there are about 0.17 milligrams of lithium in every liter of seawater.
It’s so low that you’d need to treat roughly 6000 tons of seawater to get about 1 kg of lithium. [8]
But hey, don’t get discouraged! I didn’t say it would be easy, I just said we need better technology.
What are the major environmental considerations? When it comes to lithium extraction and production, there are a few things to consider.
First, water usage. Lithium extraction often involves pumping brine or groundwater from underground sources, which can lead to water scarcity and competition with whoever is nearby like the local community, agriculture, etc. But also water contamination (more on this soon),
Then, we have chemical usage. Brine processing sometimes requires the use of chemicals for separation and purification. How you handle, store, and dispose of them matters as you’d ideally want to prevent the contamination of soil, water, and air. What chemicals are these? Solvents (kerosene or alcohols to extract lithium from the brine), Acidic compounds (sulfuric acid or hydrochloric acid to adjust the pH and help separate the lithium from other elements in the brine), and Reagants (flocculants or coagulants are used to aid in the precipitation and separation of lithium compounds).
What else? We have land disruption, carbon emissions, and affected towns of people nearby.
But how do you fix all these things? Through innovation! So we need to create cheaper and cleaner extraction technologies. And oh, recycling and reusing lithium-ion batteries help, too.
You may see ads about returning your old phone or computer so if you’re curious here’s an overview of the recycling and reuse of lithium-ion batteries:
- Collection: Used lithium-ion batteries are collected from various sources, including electronic waste recycling centers, battery manufacturers, and consumer drop-off locations.
- Sorting and Inspection: The collected batteries are sorted based on their type, size, and chemistry. They undergo visual inspection to identify any physical damage or potential safety hazards.
- Discharge: The batteries are discharged to remove any remaining electrical charge, ensuring safe handling during subsequent processing steps.
- Dismantling: The batteries are disassembled, separating the different components such as the cathode, anode, electrolyte, and casing. Manual or automated processes may be used for this step.
- Recycling of Materials: Each component is processed separately to recover valuable materials. The cathode and anode materials, typically containing metals like lithium, cobalt, nickel, and manganese, can be extracted through various techniques such as hydrometallurgical or pyrometallurgical processes.
- Purification and Refining: The recovered materials undergo purification to remove impurities and contaminants. This step aims to obtain high-quality metals that can be used in the production of new batteries or other applications.
- Reuse or Manufacturing: Depending on the condition and quality of the recovered materials, they can be reused directly in battery manufacturing after appropriate testing, and validation. Alternatively, the materials can be sold to manufacturers for the production of new batteries or used in other industries.
Alright, that's that. Let’s move on.
Lithium From Mining to Consumers' Hands
I'm a big fan of the systems approach when it comes to understanding stuff. It's like putting together a puzzle, where each piece contributes to the big picture. And guess what? Sometimes the most groundbreaking innovations come from having that deep context about how all the pieces fit and work together.
Let me break it down for you, step by step, or you can call it the systems approach to the lithium refining process. We're going to start right from the exploration phase, where they're out there searching for this valuable resource. Then, we'll follow the journey all the way until you finally get your hands on an electric phone or just a regular one.
- Exploration and Mining: The search for lithium-rich sources begins, and upon discovery, miners extract lithium-bearing ores or minerals.
- Ore Processing: The mined lithium-bearing ore undergoes various techniques like crushing, grinding, and separation to obtain a concentrated lithium-rich material.
- Extraction of Lithium Compounds: The concentrated lithium-bearing material is further processed to extract lithium compounds using methods like hydrometallurgical extraction, pyrometallurgy, or direct lithium extraction.
- Purification: The extracted lithium compounds contain impurities that require removal. Purification processes, including precipitation, filtration, and chemical treatments, refine the lithium compounds to achieve high purity.
- Conversion into Lithium Carbonate or Lithium Hydroxide: The purified lithium compounds, often in the form of lithium chloride, undergo further processing to be converted into commercially useful forms like lithium carbonate or lithium hydroxide, commonly employed in lithium-ion battery production.
- Battery Material Production: The refined lithium compounds become a key component in the production of lithium-ion batteries. They are combined with cathodes, anodes, and electrolytes to create battery cells.
- Battery Manufacturing: Battery cells, along with other components, are carefully assembled into battery packs or modules. This crucial step involves precise engineering and quality control to ensure battery performance and safety.
- Integration into Products: The manufactured lithium-ion batteries are integrated into various products, including electric vehicles, portable electronics, and energy storage systems.
Certain steps or processes may vary depending on the source of lithium (ore or brine deposits) and the desired final product.
Lithium Mining
Where exactly is lithium mined from? There are several deposit types and we will explore each one of them.
- Lithium brine deposits: These are the most common type. They're found in places where water has evaporated, leaving behind concentrated lithium salts. You can find brine deposits in Chile, Argentina, Bolivia, and the United States.
- Pegmatite deposits: Less common, but they often have higher lithium concentrations. Pegmatites are formed when molten rock cools and crystallizes. They can be found in places like Australia, Canada, and the United States.
- Sedimentary deposits: The least common type, formed when lithium-rich minerals are deposited in sedimentary rocks. You can find these deposits in China, Russia, and the United States.
Oh, wait, there's more!
- Clay deposits: Some clay minerals, like hectorite and jadarite, also contain lithium. These deposits are found in specific geological formations and may need special techniques for extraction.
- Geothermal brines: In certain geothermal areas, hot water brines carry dissolved lithium salts. While they're not a major source of lithium, they're often found in volcanic regions.
Lithium Extraction
Where Do Lithium, Anode and Cathode Materials, and Other Materials in Lithium-Ion Batteries Come From?
We understand the necessary components required for lithium-ion cells, but how do we refine and prepare them to be assembled into a functional battery?
First, we need to obtain lithium! We can source lithium from either ore deposits or brine deposits. Now, let me explain what "brine" is—it's a high-concentration solution of salt, often composed of sodium chloride or calcium chloride, dissolved in water.
When it comes to extracting lithium from ore deposits, the process involves mining and crushing the ore. Afterward, physical and chemical techniques are utilized to concentrate and purify the lithium. On the other hand, for brine deposits, we pump the brine to the surface and employ various processes to remove impurities and concentrate the lithium.
What about anode and cathode materials?
The anode, typically made of graphite, can be sourced from either natural graphite or synthetic graphite. To refine graphite, it goes through a series of steps. First, the graphite ore is crushed and ground, then subjected to flotation and other purification processes to remove impurities, resulting in a high-purity graphite powder.
As for the cathode, it usually consists of lithium metal oxides, such as lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), or lithium nickel manganese cobalt oxide (LiNiMnCoO2). To refine the cathode materials, the desired metal oxide is synthesized through chemical reactions, followed by grinding and processing to achieve the desired particle size and purity.
Anything else?
Besides anode and cathode materials, other components in lithium-ion cells, like electrolytes and separators, also undergo refining processes. Electrolytes usually consist of lithium salts dissolved in either organic solvents or solid polymer matrices.
You might be wondering, what is a solid polymer matrix? To put it simply, it's like a big ball of clay. Imagine this clay as the polymer matrix. Now, visualize poking sticks into the clay; these sticks act as the reinforcement material. In this analogy, the clay represents the polymer, which is a type of plastic, and the sticks represent various materials like fibers, particles, or flakes. The polymer matrix holds the reinforcement material in place, making it stronger and stiffer, just like in solid polymer matrices used in electrolytes.
What about separators? What are those?
They literally separate the anode and cathode to prevent a short circuit and contribute to thermal stability and safety.
To be more specific, separators are thin polymeric membranes, which are made of polymers—a type of plastic composed of repeating units. Think of them as similar to the membranes used in water purification. These separators are placed between the positively charged anode and the negatively charged cathode within the lithium-ion cell.
The primary function of separators is to keep the anode and cathode separate, ensuring there is no direct connection between their positive and negative terminals. This separation is what effectively prevents electrical short circuits from occurring. A short circuit is when there is an unintended direct connection between the positive and negative terminals of a battery, which can lead to overheating, fires, or even explosions.
Brine or Ore?
Let’s talk about how we get that precious lithium out of the Earth! Back in the day, we had to dig into tough rocks to find it, but then things changed. Nature gave us a secret: brine deposits. These are like mineral springs or pools filled with lithium-rich salt. Places like Chile, Argentina, and Bolivia are major sources of this treasure now.
Imagine yourself in Australia or North Carolina, mining hard rocks like spodumene to free the valuable lithium from them. But there's another cool way: lithium hidden in salty brine. In Chile's Salar de Atacama, the brine is gathered in ponds, basking in the sun's warmth. Patience is key as it takes time for the lithium content to reach its peak.
When the moment comes, the brine heads to the coast, where it undergoes a magnificent transformation into pure lithium carbonate, lithium hydroxide, and lithium chloride. These are the magic ingredients used in batteries and other industries.
As technology advances, we keep looking for new ways to extract lithium. How about using geothermal brine water? Passing it through a special electrolytic cell with a membrane could unlock not just lithium but other valuable minerals too.
And hold on to your hats! Scientists dream of extracting lithium from seawater through electrodialysis and electrochemical intercalation. Imagine having lithium hidden in every millionth part of the vast sea, waiting to be collected!
The quest for lithium never stops. Whether we're mining hard rocks, harnessing the power of brine pools, or dreaming of extracting it from the mighty sea, we're always searching for more. With every new idea, technology, and adventure, we're getting closer to unleashing the full potential of lithium.
If we talk about lithium extraction, we must talk about brine and ore the two main methods of extracting lithium from its natural deposits. Brine mining involves extracting lithium from underground brine reservoirs, while ore mining involves extracting lithium from lithium-bearing ores.
Here’s a table:
| Characteristic | Brine Deposits | Ore Deposits | | -------------------------------- | ------------------------------------------------- | ----------------------------------------------------- | | Formation | Formed when water evaporates, leaving concentrated lithium salts | Solid materials found in rocks or sediments | | Examples | Salar de Atacama (Chile), Salar del Hombre Muerto (Argentina), Clayton Valley (USA) | Greenbushes (Australia), Bikita (Zimbabwe), Tanco (Canada) | | Lithium Concentration | Generally lower concentration of lithium compared to ore deposits | Often higher concentration of lithium | | Extraction Method | Extracted from underground reservoirs or salt flats using evaporation ponds or chambers | Mined using conventional mining methods | | Cost and Ease of Extraction | Easier and lower cost extraction process | More challenging and higher cost extraction process | | Impurities | May contain impurities like magnesium and calcium | May contain impurities depending on the specific minerals | | Geography | Found in regions like South America and the USA | Found in various regions worldwide | | Uses and Applications | Widely used for lithium production in recent years | Historically used as the main source of lithium | | Notable Producing Countries | Chile, China, Argentina, USA | Australia, Canada, Zimbabwe, Russia, etc. | | Importance in Lithium Production | Increasingly important due to ease of extraction and economic viability | Still significant but becoming less dominant |
But we need to process it!!!
Let’s explore the nitty-gritty of brine and ore processing.
Alright, now let's compare brine processing with ore processing. They're two different methods of extracting lithium, and each comes with its own perks and quirks. Here's what sets them apart:
Brine Processing:
-
Source: In brine processing, we're after lithium in lithium-rich brine solutions found in underground reservoirs or salt flats. These brine sources contain dissolved lithium salts, just waiting to be collected.
-
Extraction: The primary method we use here is evaporation. We pump the brine into large evaporation ponds, where it basks in the sun and wind, letting the water evaporate. As the water disappears, the lithium concentration rises, and we end up with precious lithium compounds like lithium carbonate or lithium chloride.
-
Impurities: We've got to be careful with impurities. Brine processing includes steps to remove them, like magnesium, calcium, and other elements that could mess with our lithium extraction and end up in the final product.
-
Energy Requirements: Good news - brine processing is a bit gentler on the energy front compared to ore processing methods.
Ore Processing:
- Source: Ore processing involves extracting lithium from mineral ores, like spodumene, petalite, or lepidolite. These ores contain lithium-bearing minerals, and we have to get them out from the rocky embrace.
- Extraction: It's a more involved process here. First, we mine the ore and then subject it to physical and chemical treatments to concentrate and purify the lithium-bearing minerals. That can include crushing, grinding, and various separation techniques. After that, we go through roasting and acid leaching to convert the lithium-bearing minerals into a more soluble form for extraction.
- Purification: Once we've extracted the lithium compounds from the ore, we have to further process and purify them to meet the specific requirements of the end product, like lithium carbonate or lithium hydroxide.
- Energy Requirements: Ore processing tends to demand more energy compared to the gentler brine processing methods.
Both methods have their pros and cons. Brine processing is easier and often results in higher lithium recoveries, but ore processing allows us to access lithium resources more widely across different regions. So, we choose the best method based on the specific characteristics of the lithium source, extraction costs, and what we need the lithium for in the end.
Lithium Refinement
Brief History of Lithium Refinement
The history of lithium refinement is actually not as extensive as one might imagine.
Lithium was discovered in 1817 by chemist Johan August Arfwedson while analyzing petalite ore. He had a “big brain” moment and found a new alkali metal which he named Lithium after the Greek word “lithos” meaning stone.
Initially, lithium was primarily obtained from HARD ROCK in minerals like lepidolite and spodumene. Don’t get scared, they’re just fancy terms from minerals or hard rock that formed in the ground over a long ass time.
So, people would find these hard rocks and they would crush and crush until they grind the ore. But that wasn’t all. Then, they would roast the shit out of it to convert lithium-containing minerals into a mixture that contains lithium and another element like chloride that can dissolve in water.
This mixture is known as soluble lithium compounds, which is obtained after roasting the lithium ore. By the way, these compounds are essential, such as lithium hydroxide (LiOH), as they are used to produce cathode material for lithium-ion batteries.
But I gotta be honest with you. This was tough. There had to be a better way. So humans used their minds to think hard and came up with a better method: Electrolytic Extraction.
Aren’t hard-working humans great?
In the late 19th century, researchers made significant progress by developing electrolytic methods for extracting lithium from minerals.
These methods involved dissolving lithium compounds in appropriate solvents and subjecting them to electrolysis, a process that uses electricity to separate elements from each other. Electrolytic extraction, or electrolysis, is a term you might have heard before, possibly from a middle school biology lab. For instance, in the case of water, electrolysis can split water (H2O) into hydrogen (H) and oxygen (O). [9].
Electrolytic Extraction is quite interesting so let’s stop there for a second, and we’ll continue.
Electrolytic Extraction
In the case of lithium, electrolytic extraction is commonly used to isolate lithium metal from compounds such as lithium chloride (LiCl) or lithium carbonate (Li2CO3). The process involves the following steps:
- Electrolyte Preparation: An electrolyte is prepared by dissolving lithium compound(s) in a suitable solvent or molten salt. For example, lithium chloride can be dissolved in a solvent like water to form an electrolyte solution.
- Electrolysis Cell Setup: An electrolysis cell is set up consisting of two electrodes—an anode (positive electrode) and a cathode (negative electrode). The electrodes are typically made of inert materials such as graphite or platinum.
- Electrolysis Process: The electrolyte solution is placed in the the electrolysis cell, and a direct current (DC) is applied to the electrodes. The positive terminal of the power source is connected to the anode, and the negative terminal is connected to the cathode.
- Ion Migration: As the electric current passes through the electrolyte, lithium ions (Li+) from the lithium compound migrate toward the cathode, while the negative ions from the compound move toward the anode.
- Reduction at Cathode: At the cathode, the lithium ions gain electrons and undergo reduction, resulting in the deposition of metallic lithium (Li) on the cathode surface. This is the desired product of the electrolytic extraction process.
- Oxidation at Anode: At the anode, the negative ions from the lithium compound undergo oxidation, releasing electrons to the external circuit. This helps maintain the flow of electric current in the cell.
- Collection and Refining: The deposited lithium metal on the cathode is collected and further refined to obtain pure lithium metal. This may involve processes such as melting, purification, and shaping into desired forms for specific applications.
Electrolytic extraction is a highly beneficial and controlled method for obtaining pure lithium metal from its compounds. When manufacturing lithium batteries, achieving high purity levels is crucial, and electrolytic extraction provides an ideal solution for this purpose.
Back to the History of Lithium Refinement
After the initial discovery of lithium, we progressed to extracting it from minerals, and later, we developed a more ingenious method known as electrolytic extraction. But the story of lithium refinement did not end there!
So, what came after?
In the 20th century, there was a notable shift in lithium production, with a focus on brine deposits, which contain abundant lithium-containing salts. These large brine deposits, found in countries like Bolivia and the United States, emerged as significant sources of lithium. The production process entailed pumping brine into evaporation ponds, where solar evaporation allowed the water to evaporate, leaving behind purified lithium extracted from the brine and ores.
And more progress came!
Advancements in processing technologies have improved the efficiency and purity of lithium refinement. Techniques like solvent extraction, ion exchange, and membrane filtration have been used to extract and purify lithium from brines and ores.
Lithium demand wasn’t that high for decades but then the lithium battery as well as the sustainability movement came and everyone started working super hard to meet that demand.
The rise of lithium-ion batteries for applications like electric vehicles or portable electronics contributed to the significant increase in lithium demand in recent decades. This demand led to an increased in the exploration and production of lithium worldwide.
Ok, that was a brief history of lithium refinement. I want to learn more about the methods of refinement.
Methods of Lithium Refinement
Alright, here's a rundown of some methods used in lithium refinement:
-
Solvent Extraction: It's like a cool way to separate specific stuff from a solution using an organic solvent. So for lithium, we mix the solution with the solvent that's really into lithium ions. The lithium ions move from the solution to the solvent, forming a separate phase. This lets us separate the lithium from the rest of the solution, and then we can further process the solvent to get purified lithium compounds.
-
Ion Exchange: In this process, we use a special solid material called an ion exchange resin. It's like a magnet for lithium ions. When the solution flows through the resin, the lithium ions stick to it, and other ions get released. We can then get the lithium ions back from the resin by treating it with something special, which helps us separate and purify them.
-
Membrane Filtration: We have this semi-permeable membrane that lets certain stuff through while blocking others. So for lithium, we use reverse osmosis or nanofiltration. The membrane has tiny pores that allow smaller lithium ions to pass while keeping larger ions and impurities out. By applying pressure, the lithium-rich solution is collected on one side, leaving the impurities behind.
...And there's more! Here are some other methods used for lithium processing:
-
Calcination: We heat up lithium-containing stuff to high temperatures to get rid of impurities and make it more reactive for further processing.
-
Acid Leaching: This involves treating lithium-containing ores with acids to dissolve the lithium compounds, making it easier to separate and purify them.
-
Precipitation: Here, we control the pH and other factors to form insoluble lithium compounds that we can then filter out from other elements.
-
Evaporation: Used in brine processing, we concentrate the lithium-rich brine by evaporating the water, which increases the lithium concentration for easier extraction.
-
Crystallization: By carefully controlling temperature, pressure, and other stuff, we can selectively crystallize and separate the desired lithium salts in a purified form.
So these methods are all part of lithium processing, and each has its own advantages and challenges. The choice of technique depends on factors like cost, efficiency, and environmental concerns, as well as the type of lithium source being used (brines or ores) and the specific end products we want to get.
Precision and Purity of Lithium
Battery grade lithium refers to lithium that has been refined to meet stringent purity standards specifically required for battery applications. In the context of lithium-ion batteries, which are widely used in portable electronics, electric vehicles, and energy storage systems, the quality and purity of lithium are crucial for optimal battery performance and longevity.
When it comes to battery-grade lithium, the term "ultra-pure" signifies an exceptionally high level of purity. Any impurities present in lithium can have detrimental effects on the performance and lifespan of the battery. Even minute amounts of impurities can cause degradation and impact the overall efficiency of the battery system.
To ensure the desired level of purity, the refinement process for battery-grade lithium involves advanced techniques and technologies. These processes aim to eliminate or reduce impurities such as metal contaminants, moisture, and other undesirable substances. The resulting lithium must meet strict specifications regarding its chemical composition, crystal structure, and overall purity.
The production of ultra-pure lithium requires a combination of mining, extraction, and refining processes. Various methods, including chemical processes, precipitation, and filtration, are employed to achieve the necessary purity levels. Advanced purification technologies, such as solvent extraction and electrolysis, are often utilized to further enhance the purity of lithium.
Maintaining a high degree of purity in battery-grade lithium is essential for several reasons. First and foremost, it ensures the stability and reliability of the battery. Impurities can react with the other components of the battery, leading to the formation of unwanted byproducts or detrimental chemical reactions that can degrade the performance of the battery over time.
Furthermore, ultra-pure lithium helps minimize the risk of side reactions, such as the formation of dendrites, which can cause short circuits and compromise the safety of the battery. Dendrites are tiny crystalline structures that can grow within the battery and bridge the electrode surfaces, leading to internal shorts and potential thermal runaway.
The demand for battery-grade lithium has grown significantly in recent years, driven by the increasing adoption of lithium-ion batteries in various industries. As a result, efforts to improve lithium refining processes and enhance the purity of battery -rade lithium are ongoing. Innovations in refining technologies, such as the development of more efficient purification methods and improved quality control measures, are being explored to meet the growing demand for ultra-pure lithium.
Ultra-pure battery-grade lithium is a key component in the production of high-performance lithium-ion batteries. Its ultra-high purity levels ensure the stability, efficiency, and safety of the battery system. The continuous advancements in lithium refinement techniques are vital for meeting the exacting standards required by the battery industry and enabling the development of next-generation energy storage solutions.
Starting a Lithium Refinery Company
If I were to start a Lithium Refinery company, here’s what I’d do.
Goal: Establishing The Galtian Refinery Line - The World's Leading Lithium Refinery Company
What do I need to do?
- Get Lithium
Finding reliable sources of lithium is crucial. We’'ll explore various methods, including partnerships with mining companies, extraction from existing lithium-rich locations, or innovative technologies like lithium recovery from brines and geothermal sources.
- Refining
Distinguishing ourselves through superior refining techniques is essential. We'll invest in research and development to optimize the refining process, seeking ways to reduce energy consumption, minimize waste, and increase purity levels.
- Sell it O ur selling process will be extremely simple. We will focus on targeting specific customer(s) to simplify our process and remove unnecessary steps from our production focus. More on why that’s important and a key innovation later.
How We’re Unique
- How We Get the Lithium
Strategic Location: Selecting an ideal location near lithium mines or deposits will reduce transportation costs and enhance supply chain efficiency. Additionally, setting up the refinery close to battery manufacturers or end consumers will streamline distribution.
- How we Refine Lithium
Tailored Refining: Customizing our refining processes to cater to specific customer needs will be a differentiator. We'll collaborate closely with battery makers, Tesla, and other potential clients to provide lithium products that meet their unique requirements.
Efficient Purification Techniques: Focus on improving the efficiency and effectiveness of purification processes for lithium compounds. This could involve the development of advanced separation technologies, such as improved membrane filtration or selective crystallization techniques, to obtain high-purity lithium hydroxide with reduced energy and chemical inputs.
Continuous Innovation: A culture of innovation will be fostered within the company. We'll constantly experiment with new refining methods, explore eco-friendly energy sources (like nuclear reactors), and invest in sustainable practices. The two most important parts of our innovation process are 1) Removing steps: thinking about our processes from first principles, and asking what really needs to be done to satisfy our consumers, and 2) Trying new things: we plan to run this company as a startup so trying out the latest technologies and merging them with existing processes will us the window of opportunity and edge to beat any competition.
I want to elaborate on the removing steps part. It sounds obvious but it’s really not. In the example of electric car batteries, car manufacturers need ultra-precise and ultra-pure lithium to avoid impurities that can degrade battery performance. Lithium hydroxide is one of the most efficient forms of lithium for battery applications so what lots of refining companies end up doing is converting lithium hydroxide to lithium carbonate, and then back to lithium hydroxide. Why? Flexibility and market demand. Different industries and battery manufacturers have varying requirements for the type of lithium compound they need, either lithium hydroxide or lithium carbonate, depending on their specific battery chemistry and design.
But if we focus on specific car manufacturers, we can become their sole source of Lithium so we reduce our costs, our processes are more efficient, and the customer is happier. If the customer(s) is large enough, we’d reach economies of scale faster, making everyone happier. This is one example of how we’d tackle this industry.
-
Consumer-centric: as stated above.
-
Waste and Recycling Utilization: Any byproducts or unused materials will not go to waste. We'll explore opportunities to sell or reuse them, ensuring a circular and sustainable economy. Establishing efficient recycling processes not only helps reduce the reliance on new lithium production but also enables the extraction of high-quality lithium compounds from recycled sources, potentially lowering costs and environmental impact.
-
Sustainability Focus: Emphasizing sustainability and eco-friendliness will not only align with global trends but also attract environmentally-conscious customers and investors. No by-products should harm nearby communities or the environment as a whole.
-
Energy Optimization: Explore ways to optimize the energy consumption of the overall refining process. This could involve integrating renewable energy sources, adopting energy-efficient technologies, and implementing process optimization strategies to minimize energy requirements and reduce operational costs.
-
Extraction: We don’t intend to get into the extraction business but we might see ourselves get into that business to improve our business. Here are a few ways:
-
Advanced Lithium Extraction: Explore and optimize novel methods for extracting lithium from brine or ore deposits. This could involve the use of innovative solvents, selective ion exchange resins, or advanced membrane technologies that enhance lithium recovery while minimizing energy consumption and environmental impact.
-
Direct Lithium Extraction: Investigate and develop direct lithium extraction technologies that allow for the selective extraction of lithium from brine without the need for evaporation ponds. Direct extraction methods, such as adsorption or selective precipitation, could significantly reduce processing time and costs associated with brine concentration and impurity removal.
-
Quality Assurance: Establishing stringent quality control measures to deliver consistently high-quality lithium products will build trust and credibility in the market. We would have an edge as we’d be more specialized and targeting specific customers so we wouldn’t need multiple quality checks for different kinds of Lithium.
-
Our Name: Whoever recognizes the name and likes it would probably be more inclined to do business with us. If you know, you know.
Let’s Make Lithium:
This is my plan to make The Galtian Refinery Line, the world's leading lithium refinery company.
Summers of Obsessions
This is the second summer in a row where I wake up every day and spend at least thirty to ninety minutes obsessing over a topic.
Last year, it was all about flying things. This summer, my mind has been consumed by Lithium.
Writing about an obsession over a period of a few months is both exciting and painful. The excitement comes from the initial thirst for knowledge, waking up at 4 AM, spending hours in the quiet morning to learn and learn. But at times, it's also painful. There's so much I don't know or other ideas and projects calling my attention.
Summers of Obsessions has become my way of life. Now that I reflect on it, two summers ago, I was obsessed with Affordable Profitable Housing.
Even the summer before that, I was fixated on the future of marketing using neuroscience principles and wrote an entire book with a neuroscience professor.
That is how without even realizing Summers of Obsessions has become a habit. I find an idea that excites me, conspire secretly to uncover cool concepts for a company or project, get into into online research, talk to anyone who's willing to engage, and use writing as my medium.
On May 18th, 2023, I started this document which became this book. Two months later, I had learned “everything” I wanted to learn about Lithium.
What will come out of this project? I have no idea and that's the beauty of it. I'm not attached to any outcome. I'm grateful for the opportunity I had to spend a summer asking questions about lithium, and really anything I wanted to learn, and then writing about it.
We are naturally curious beings. We are born with a desire to learn and explore. But as we grow older, we lose that curiosity. We become more concerned with what others think of us, and we stop asking questions. Doing a project as The Cheesy Energy of Lithium is my way of keeping that curiosity alive and well.
It all starts with a question, curiosity is the fuel, and writing is the medium.
If you'd like to keep in touch and learn more about my projects, join my weekly memos here.
Other Random Curiosities About Lithium
- Lithium Tokamak Experiment
- Lithia Water (itself, company)
- U.S. Lithium Infrastructure:
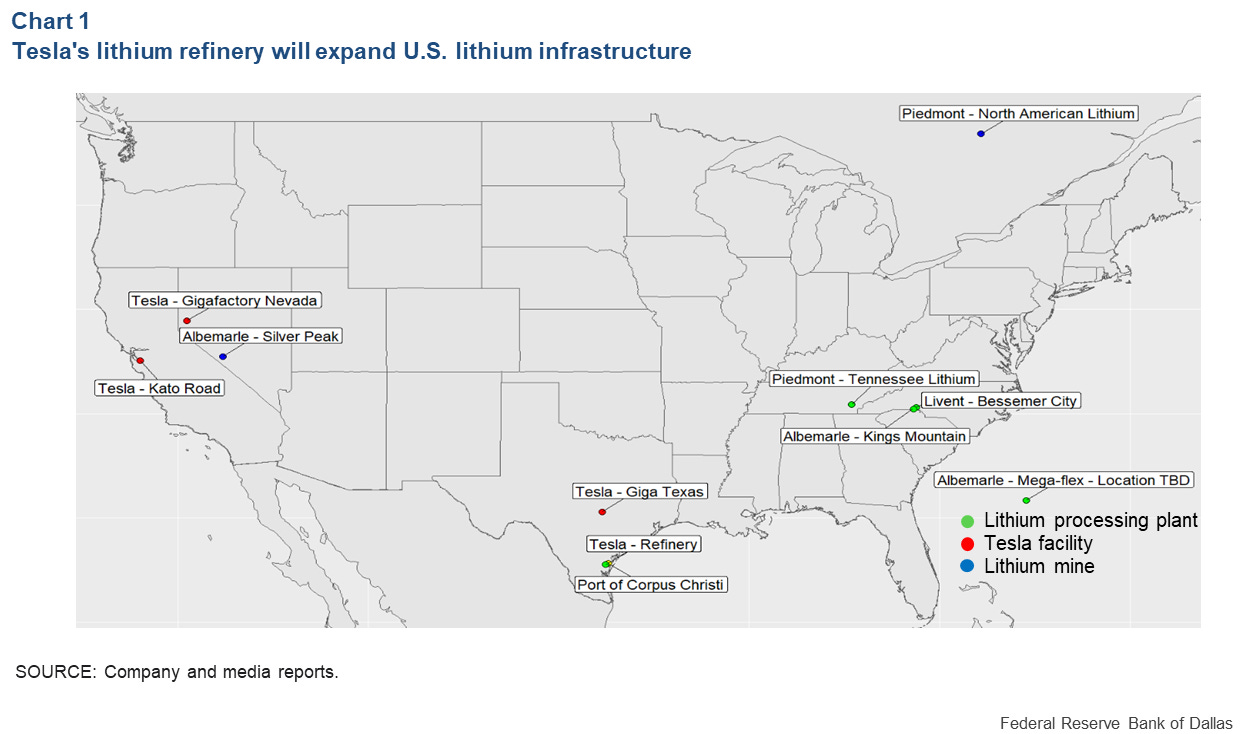
Good Resources
Questions
- How can we use deep knowledge of theoretical physics to refine lithium better?
- How can we use deep knowledge of theoretical physics to find better ways to imagine the world? What stories?
- How do we create the ideal battery?
If I made a mistake anywhere, please let me know or tweet at me.
Notes
[1] Radioactive decay isn’t solely caused by unbalanced protons or neutrons. Various conditions can contribute to radioactive decay:
-
Excess of Nucleons: When the nucleus of an atom contains an abundance of protons and neutrons, it becomes destabilized. Consequently, the atom seeks a more stable configuration by releasing particles or radiation.
-
Nuclear Excitation: Nuclei can be excited into higher energy states through various processes, such as particle collisions or the absorption of energy. These excited states are often even more unstable, leading the nucleus to undergo radioactive decay to return to a more stable state.
-
High Atomic Number: Elements with higher atomic numbers tend to have more unstable isotopes and are more likely to undergo radioactive decay. As the atomic number increases, the delicate balance between protons and neutrons becomes more precarious, resulting in increased instability.
-
Neutron-Induced Decay: Certain isotopes undergo decay when they absorb an additional neutron. The extra neutron may render the nucleus unstable, prompting it to undergo radioactive decay to achieve a more stable and balanced state.
-
External Influences: High-energy radiation or particle bombardment can trigger or induce radioactive decay in certain isotopes.
[2] Am I the only one who feels like this sounds straight out of a Marvel movie?
In beta-plus (β+) decay, a proton transforms into a neutron, emitting a positron (positively charged beta particle) and a neutrino.
A positron is the antimatter counterpart of an electron. When a positron encounters an electron, they annihilate each other, releasing energy in the form of gamma rays.
[3] Positron emission and electron capture are related to beta decay as they involve the capture or emissions of electrons or positrons.
Positron Emission: Positron emission occurs when a proton-rich nucleus undergoes radioactive decay. In this process, a proton in the nucleus is converted into a neutron, and a positron (a positively charged electron) and a neutrino are emitted. Positron emission is associated with beta-plus (β+) decay.
Electron Capture: Electron capture is a process where an inner orbital electron is captured by the nucleus. This captured electron combines with a proton to form a neutron and a neutrino is emitted. Electron capture is associated with beta-minus (β-) decay.
[4] Various cathode materials can be utilized in batteries, offering different properties to meet specific requirements. Some common cathode materials include lithium cobalt oxide, lithium nickel manganese cobalt oxide, and lithium iron phosphate. The choice of cathode material depends on the battery's needs concerning energy density, power output, and safety considerations. Each material offers distinct advantages, allowing manufacturers to tailor batteries for various applications, whether it be high energy storage, rapid power delivery, or enhanced safety features. The versatility of cathode materials plays a crucial role in the advancement of battery technology and its diverse applications in the market.
[5] In 1936, an intriguing artifact was discovered in Iraq, but its true purpose remained a mystery. Some speculated that it might have been used for electrotherapy, but there was no definitive answer. However, the artifact's fate took a puzzling turn during the Iraq War in 2003 when it disappeared, and its whereabouts have remained unknown ever since. The enigmatic journey of this artifact leaves us with more questions than answers, adding to its mystique and allure.
[6] Backhaus R. Battery Raw Materials - Where from and Where to? ATZ Worldw. 2021;123(9):8–13. doi: 10.1007/s38311-021-0715-5. Epub 2021 Aug 27. PMCID: PMC8390110.
[7] Abundances of the elements (data page)
[8] Dang C, Helal AS, Zhu L, et al. Industrial pathways to lithium extraction from seawater: Challenges and perspectives. Nano Research Energy, 2023, 2: e9120059. https://doi.org/10.26599/NRE.2023.9120059
[9] If you’re into chemistry, you probably screamed when you saw the wrong chemical formula.
What’s the actual chemical formula for the electrolysis of water?
2H2O → 2H2 + O2
You may ask, “H2O is water. I knew that much. But why is there a 2 in front of the H2O?”
The 2 in front of H20 in the equation for the electrolysis of water tells you that two water molecules are required to produce one oxygen molecule and two hydrogen molecules.
Why? The water molecule (H2O) is made up of two hydrogen atoms and one oxygen atom. When an electric current is passed through water, the hydrogen atoms are attracted to the negative electrode, where they combine to form hydrogen gas.
While, the oxygen atoms are attracted to the positive electrode, where they combine to form oxygen gas.
So why am I writing this? BECAUSE it’s IMPORTANT and because I was curious and did not know myself. The 2 in front of H2O is important to write when doing the electrolysis because it ensures the correct amount of water is used.
If too little water is used, then not enough oxygen gas will be produced. If too much water is used, then the process will be inefficient and more energy will be required.
Here’s a recap of the electrolysis of water:
- An electric current is passed through water.
- The hydrogen atoms are attracted to the negative electrode, where they combine to form hydrogen gas.
- The oxygen atoms are attracted to the positive electrode, where they combine to form oxygen gas.
- Hydrogen and oxygen gas are collected at the electrodes.
Electrolysis of water is actually pretty cool and you can use it to produce hydrogen gas, oxygen gas, and other chemicals. This is how chlorine and caustic soda are produced.
The attentive reader will ask, “Hold on a second. Why do we write 2H2O for the electrolysis of water and not for regular H2O?
In the case of electrolysis, the water molecule will be split!!! And in that case, you need two water molecules are required to produce one oxygen molecule and two hydrogen molecules.
The number 2 in front of the 2H2O chemical formula tells you that there are two molecules of water. The 2 multiplies the number of atoms of each element in the chemical formula. For example, 2H2O represents four H atoms and two O atoms.
So far, so good.
The super attentive reader will ask, “Thanks, Juan David. But why are hydrogen atoms attracted to the negative electrode, and why are the oxygen atoms attracted to the positive electrode?”
One word: ELECTRONEGATIVITY.
What the hell is that? Electronegativity is a measure of an atom's ability to attract electrons.
WHAT???? Let me give you an analogy.
Imagine you're hosting a party, and the guests are electrons. Electronegativity is like the party host's magnetism for attracting guests to different areas of the party.
Some party hosts have high electronegativity. They're like the life of the party, constantly pulling electrons towards themselves. It's as if they have an irresistible charm that makes electrons want to hang out with them. These hosts are like the popular kids in school who always have a crowd of followers around them.
On the other hand, some hosts have low electronegativity. They're like the shy, introverted individuals who don't attract much attention. Electrons are less drawn to them, as if they're quietly sitting in a corner, minding their own business. These hosts are like the wallflowers at the party who prefer to observe rather than be the center of attention.
Now, let's say there's a potluck at the party, and guests bring different dishes. The dishes represent the elements in the periodic table. The party host with high electronegativity is like a food magnet. Whenever there's a tasty dish brought by an electron-guest, they quickly snatch it away, leaving the other hosts with fewer options. It's as if they have a knack for grabbing the best treats at the party.
Meanwhile, the hosts with low electronegativity are more laid-back about the food. They don't aggressively seek out the dishes brought by the electrons. They're more open to sharing and enjoying a variety of dishes, even if they're not the most extravagant or popular choices.
In this analogy, electronegativity is the party host's ability to attract and hold onto the electrons (party guests) based on their charisma and preferences. The hosts with high electronegativity are like the popular, attention-grabbing individuals, while those with low electronegativity are more relaxed and less inclined to steal the show.
That’s electronegativity! But let’s continue the analogy to answer the question of why hydrogen atoms are attracted to the negative electrode and why oxygen atoms are attracted to the positive electrode.
GET READY BECAUSE WE’RE GOING TO ANOTHER PARTY!!!
Imagine that the party venue has two separate dance floors—one with a "Negative Electrode" sign and the other with a "Positive Electrode" sign.
Hydrogen atoms, being social and outgoing, are naturally drawn to the negative electrode dance floor. It's like they hear the lively music and see the energetic crowd there, and they can't resist joining in on the fun. They are like the party guests who are always ready to dance, mingle, and create a vibrant atmosphere. So, the hydrogen atoms are naturally attracted to the negative electrode, gravitating towards the excitement and high energy of that space.
On the other hand, oxygen atoms, being a bit more reserved and sophisticated, are intrigued by the positive electrode dance floor. They see the elegant decorations, the calm ambiance, and perhaps some smooth jazz playing in the background. The positive electrode dance floor has a more relaxed atmosphere that appeals to the oxygen atoms' refined taste. They feel like they can have meaningful conversations and enjoy a sophisticated evening. So, the oxygen atoms are naturally attracted to the positive electrode, seeking the serenity and elegance that it offers.
This analogy aligns with the real-world phenomenon of electrolysis, where hydrogen and oxygen are generated through the splitting of water molecules using an electric current. In this process, hydrogen gas (H2) is produced at the negative electrode (cathode), which attracts positive ions, while oxygen gas (O2) is produced at the positive electrode (anode), attracting negative ions.
By relating it back to our electronegativity analogy, we can say that hydrogen atoms, being less electronegative, are more willing to let loose and join the negative electrode dance floor, while oxygen atoms, with their higher electronegativity, prefer the refined environment of the positive electrode dance floor.
But remember the movement of ions during electrolysis is driven by the flow of electrons and the attraction of opposite charges at the electrodes.
So yeah, hydrogen atoms are attracted to the negative electrode, and oxygen atoms are attracted to the positive electrode, because of the difference in electronegativity between the two atoms. Electronegativity is a measure of an atom's ability to attract electrons. Oxygen is more electronegative than hydrogen, meaning that it has a stronger pull on electrons. This causes the electrons in the water molecule to be pulled closer to the oxygen atom, leaving the hydrogen atoms with a partial positive charge.
When an electric current is passed through water, the negatively charged electrons are attracted to the positive electrode, while the positively charged hydrogen ions are attracted to the negative electrode. This causes the hydrogen atoms to be stripped of their electrons, leaving behind hydrogen gas. The oxygen atoms, which have gained electrons, combine to form oxygen gas.
The negative electrode is called the cathode, and the positive electrode is called the anode. The cathode is where the reduction reaction occurs, and the anode is where the oxidation reaction occurs.
The reduction reaction is the process of gaining electrons, while the oxidation reaction is the process of losing electrons. In the case of the electrolysis of water, the hydrogen atoms are reduced at the cathode, while the oxygen atoms are oxidized at the anode.
In a more technical sense, the hydrogen atoms are attracted to the negative electrode because they have a lower ionization energy than the oxygen atoms. Ionization energy is the energy required to remove an electron from an atom. The lower the ionization energy, the easier it is to remove an electron from the atom.
The oxygen atoms are attracted to the positive electrode because they have a higher electronegativity than the hydrogen atoms. Electronegativity is a measure of an atom's ability to attract electrons. The higher the electronegativity, the stronger the atom's pull on electrons.
Ok, I’m done now.
I also asked my future professor friend and this is what he said:
The 2 in front of H2O in the chemical equation for electrolysis means that for every 2 water molecules, 4 hydrogen molecules and 2 oxygen gas molecules will be produced. This is important to understand because it helps us understand the mechanism of the process and how much energy is required.
The entropy of the system increases with electrolysis as more molecules are created. This is because entropy is a measure of disorder, and the more molecules there are in a system, the more disordered it is. Electrolysis can be more efficient at higher temperatures because the system favors higher entropy.
Protons (H+) are attracted to the negative electrode (cathode) and are reduced to hydrogen gas. Hydroxide ions (OH-) are attracted to the positive electrode (anode) and are oxidized to oxygen gas and water.
There are three main types of electrolyzers that can produce hydrogen gas: PEM, AEM, and alkaline electrolyzers.
PEM (polymer electrolyte membrane) electrolyzers are the most common type of electrolyzer. They use a polymer membrane to separate the electrodes, which makes them less expensive than other types of electrolyzers.
AEM (anion exchange membrane) electrolyzers are similar to PEM electrolyzers, but they use an anion exchange membrane instead of a polymer membrane. This makes them more efficient at higher temperatures, but they are also more expensive.
Alkaline electrolyzers are the oldest type of electrolyzer. They are less efficient than PEM and AEM electrolyzers, but they are also less expensive.
Thank you, future professor.
Thank you for reading, The Cheesy Energy of Lithium. If you enjoyed it, please share it with a friend or two!
If you’re into interesting ideas (like the one you just read), join my Weekly Memos, and I’ll send you new essays right when they come out.